当前位置:
X-MOL 学术
›
Asian J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Formal [4+1] Cyclization of ortho- or para-Quinone Methides with 3-Chlorooxindoles: Synthesis of 3,2′-Tetrahydrofuryl Spirooxindoles
Asian Journal of Organic Chemistry ( IF 2.7 ) Pub Date : 2021-08-10 , DOI: 10.1002/ajoc.202100363 Xiaochen Tian 1 , Xiaoli Zhang 1 , Xiaohan Hou 1 , Weiwu Ren 1, 2 , Xiaoyang Li 1 , Fei Zhao 3 , Houchao Tao 3 , Yang Wang 1, 2
Asian Journal of Organic Chemistry ( IF 2.7 ) Pub Date : 2021-08-10 , DOI: 10.1002/ajoc.202100363 Xiaochen Tian 1 , Xiaoli Zhang 1 , Xiaohan Hou 1 , Weiwu Ren 1, 2 , Xiaoyang Li 1 , Fei Zhao 3 , Houchao Tao 3 , Yang Wang 1, 2
Affiliation
|
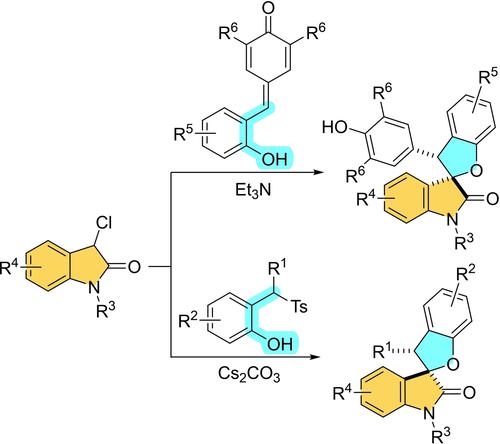
A base promoted formal [4+1] cycloaddition of ortho- and para-quinone methides with 3-chlorooxindoles is reported to afford various functionalized 3,2’-tetrahydrofuryl spirooxindoles with an unexpected reversal diastereoselectivity. Different configurations of 1,4-addition and 1,6-addition at the oxindole carbanionic center are found to the origin of diastereoselection through DFT calculations. These structurally unique spirooxindole derivatives show promising anti-tumor activity.

中文翻译:

邻-或对-醌甲基与 3-氯氧吲哚的正式 [4+1] 环化:合成 3,2'-四氢呋喃螺氧吲哚
据报道,碱促进了邻- 和对 -醌甲基化物与 3-氯代吲哚的正式 [4+1] 环加成反应,以提供各种功能化的 3,2'-四氢呋喃基螺吲哚,并具有意想不到的逆转非对映选择性。通过 DFT 计算,发现 oxindole 碳负离子中心的 1,4-加成和 1,6-加成的不同构型是非对映选择的起源。这些结构独特的螺吲哚衍生物显示出有希望的抗肿瘤活性。

更新日期:2021-09-13

中文翻译:

邻-或对-醌甲基与 3-氯氧吲哚的正式 [4+1] 环化:合成 3,2'-四氢呋喃螺氧吲哚
据报道,碱促进了邻- 和对 -醌甲基化物与 3-氯代吲哚的正式 [4+1] 环加成反应,以提供各种功能化的 3,2'-四氢呋喃基螺吲哚,并具有意想不到的逆转非对映选择性。通过 DFT 计算,发现 oxindole 碳负离子中心的 1,4-加成和 1,6-加成的不同构型是非对映选择的起源。这些结构独特的螺吲哚衍生物显示出有希望的抗肿瘤活性。




























 京公网安备 11010802027423号
京公网安备 11010802027423号