当前位置:
X-MOL 学术
›
J. Biochem. Mol. Toxicol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
SNHG5 protects chondrocytes in interleukin-1β-stimulated osteoarthritis via regulating miR-181a-5p/TGFBR3 axis
Journal of Biochemical and Molecular Toxicology ( IF 3.6 ) Pub Date : 2021-08-08 , DOI: 10.1002/jbt.22866 Yang Yue 1 , Sun Zhibo 1 , Liu Feng 1 , Bai Yuanzhang 1 , Wu Fei 1
Journal of Biochemical and Molecular Toxicology ( IF 3.6 ) Pub Date : 2021-08-08 , DOI: 10.1002/jbt.22866 Yang Yue 1 , Sun Zhibo 1 , Liu Feng 1 , Bai Yuanzhang 1 , Wu Fei 1
Affiliation
|
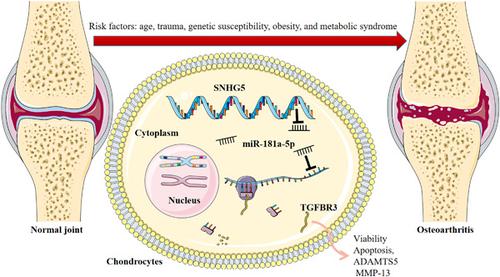
Long noncoding RNAs (lncRNAs) have been considered as important modulators in the development of osteoarthritis. The present study investigates whether there is a link between lncRNA small nucleolar RNA host gene 5 (SNHG5) and osteoarthritis pathogenesis, and the underlying molecular mechanism. To establish an in vitro model of osteoarthritis, interleukin 1β (IL-1β) was used to treat chondrocytes (C20/A4 cells) for mimicking the inflammatory condition in osteoarthritis pathogenesis. SNHG5 and miR-181a-5p expression levels were then detected in cartilage tissues of osteoarthritis patients and C20/A4 cells by quantitative polymerase chain reaction (qPCR). Cell counting kit-8 and 5-ethynyl-2ʹ-deoxyuridine assays were applied for detecting the viability of chondrocytes, and the apoptosis of chondrocytes was examined through caspase-3 activity assay and flow cytometry analysis. Western blot and qPCR were employed for determining the expression levels of TGFBR3, ADAMTS5, and MMP-13. The regulatory relationships among SNHG5, miR-181a-5p, and TGFBR3 were verified by RNA immunoprecipitation and dual-luciferase reporter assays. The expression levels of SNHG5 and TGFBR3 were markedly decreased, and miR-181a-5p expression was enhanced in osteoarthritis tissues and chondrocytes treated with IL-1β. SNHG5 knockdown inhibited the viability of chondrocytes, induced apoptosis, and promoted the expression levels of ADAMTS5 and MMP-13. Conversely, SNHG5 overexpression could counteract the effects of IL-1β, increase the viability of chondrocytes and suppress apoptosis. Mechanically, SNHG5 positively regulated TGFBR3 expression via sponging miR-181a-5p. Moreover, miR-181a-5p overexpression and TGFBR3 knockdown counteracted the effects of SNHG5 on chondrocytes. SNHG5 can probably protect chondrocytes from the inflammatory response and reduce the degradation of the extracellular matrix via modulating the miR-181a-5p/TGFBR3 axis.
中文翻译:

SNHG5 通过调节 miR-181a-5p/TGFBR3 轴保护白细胞介素 1β 刺激的骨关节炎中的软骨细胞
长链非编码 RNA (lncRNA) 已被认为是骨关节炎发展中的重要调节剂。本研究探讨 lncRNA 小核仁 RNA 宿主基因 5 (SNHG5) 与骨关节炎发病机制之间是否存在联系,以及潜在的分子机制。为了建立骨关节炎的体外模型,白细胞介素 1β (IL-1β) 用于治疗软骨细胞(C20/A4 细胞)以模拟骨关节炎发病机制中的炎症状况。然后通过定量聚合酶链反应 (qPCR) 在骨关节炎患者的软骨组织和 C20/A4 细胞中检测 SNHG5 和 miR-181a-5p 的表达水平。细胞计数试剂盒 8 和 5-乙炔基-2′-脱氧尿苷测定用于检测软骨细胞的活力,通过caspase-3活性测定和流式细胞术分析检测软骨细胞的凋亡。Western印迹和qPCR用于确定TGFBR3、ADAMTS5和MMP-13的表达水平。SNHG5、miR-181a-5p 和 TGFBR3 之间的调控关系通过 RNA 免疫沉淀和双荧光素酶报告基因分析得到验证。用 IL-1β 处理的骨关节炎组织和软骨细胞中 SNHG5 和 TGFBR3 的表达水平显着降低,miR-181a-5p 表达增强。SNHG5 敲低抑制软骨细胞的活力,诱导细胞凋亡,并促进 ADAMTS5 和 MMP-13 的表达水平。相反,SNHG5 过表达可以抵消 IL-1β 的作用,增加软骨细胞的活力并抑制细胞凋亡。机械地,SNHG5 通过海绵化 miR-181a-5p 正向调节 TGFBR3 的表达。此外,miR-181a-5p 过表达和 TGFBR3 敲低抵消了 SNHG5 对软骨细胞的影响。SNHG5 可能通过调节 miR-181a-5p/TGFBR3 轴来保护软骨细胞免受炎症反应并减少细胞外基质的降解。
更新日期:2021-10-15
中文翻译:

SNHG5 通过调节 miR-181a-5p/TGFBR3 轴保护白细胞介素 1β 刺激的骨关节炎中的软骨细胞
长链非编码 RNA (lncRNA) 已被认为是骨关节炎发展中的重要调节剂。本研究探讨 lncRNA 小核仁 RNA 宿主基因 5 (SNHG5) 与骨关节炎发病机制之间是否存在联系,以及潜在的分子机制。为了建立骨关节炎的体外模型,白细胞介素 1β (IL-1β) 用于治疗软骨细胞(C20/A4 细胞)以模拟骨关节炎发病机制中的炎症状况。然后通过定量聚合酶链反应 (qPCR) 在骨关节炎患者的软骨组织和 C20/A4 细胞中检测 SNHG5 和 miR-181a-5p 的表达水平。细胞计数试剂盒 8 和 5-乙炔基-2′-脱氧尿苷测定用于检测软骨细胞的活力,通过caspase-3活性测定和流式细胞术分析检测软骨细胞的凋亡。Western印迹和qPCR用于确定TGFBR3、ADAMTS5和MMP-13的表达水平。SNHG5、miR-181a-5p 和 TGFBR3 之间的调控关系通过 RNA 免疫沉淀和双荧光素酶报告基因分析得到验证。用 IL-1β 处理的骨关节炎组织和软骨细胞中 SNHG5 和 TGFBR3 的表达水平显着降低,miR-181a-5p 表达增强。SNHG5 敲低抑制软骨细胞的活力,诱导细胞凋亡,并促进 ADAMTS5 和 MMP-13 的表达水平。相反,SNHG5 过表达可以抵消 IL-1β 的作用,增加软骨细胞的活力并抑制细胞凋亡。机械地,SNHG5 通过海绵化 miR-181a-5p 正向调节 TGFBR3 的表达。此外,miR-181a-5p 过表达和 TGFBR3 敲低抵消了 SNHG5 对软骨细胞的影响。SNHG5 可能通过调节 miR-181a-5p/TGFBR3 轴来保护软骨细胞免受炎症反应并减少细胞外基质的降解。


























 京公网安备 11010802027423号
京公网安备 11010802027423号