Bioorganic & Medicinal Chemistry ( IF 3.5 ) Pub Date : 2021-08-08 , DOI: 10.1016/j.bmc.2021.116360 Ana R Gomes 1 , Ana S Pires 2 , Ana M Abrantes 2 , Ana C Gonçalves 3 , Saul C Costa 4 , Carla L Varela 5 , Elisiário T Silva 5 , Maria F Botelho 2 , Fernanda M F Roleira 5
|
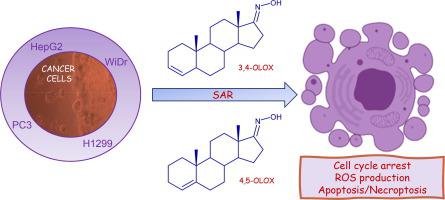
Steroidal compounds were proven to be efficient drugs against several types of cancer. Oximes are also chemical structures frequently associated with anticancer activity. The main goal of this work was to combine the two referred structures by synthesizing steroidal oximes and evaluating them in several cancer cell lines. Compounds (17E)-5α-androst-3-en-17-one oxime (3,4 – OLOX), (17E)-3α,4α-epoxy-5α-androstan-17-one oxime (3,4 – EPOX), (17E)-androst-4-en-17-one oxime (4,5 – OLOX) and (17E)-4α,5α-epoxyandrostan-17-one oxime (4,5 – EPOX) were synthesized and their cytotoxicity evaluated in four human cancer cell lines, namely colorectal adenocarcinoma (WiDr), non-small cell lung cancer (H1299), prostate cancer (PC3) and hepatocellular carcinoma (HepG2). A human non-tumour cell line, CCD841 CoN (normal colon cell line) was also used. MTT assay, flow cytometry, fluorescence and hemocompatibility techniques were performed to further analyse the cytotoxicity of the compounds. 3,4 – OLOX was the most effective compound in decreasing tumour cell proliferation in all cell lines, especially in WiDr (IC50 = 9.1 μM) and PC3 (IC50 = 13.8 μM). 4,5 – OLOX also showed promising results in the same cell lines (IC50 = 16.1 μM in WiDr and IC50 = 14.5 μM in PC3). Further studies also revealed that 3,4 – OLOX and 4,5 – OLOX induced a decrease in cell viability accompanied by an increase in cell death, mainly by apoptosis/necroptosis for 3,4 – OLOX in both cell lines and for 4,5 – OLOX in WiDr cells, and by necrosis for 4,5 – OLOX in PC3 cells. These compounds might also exert their cytotoxicity by ROS production and are not toxic for non-tumour CCD841 CoN cells. Additionally, both compounds did not induce haemoglobin release, proving to be safe for intravenous administration. 3,4 – OLOX and 4,5 – OLOX might be the starting point for an optimization program towards the discover of new steroidal oximes for anticancer treatment.
中文翻译:

甾体肟的设计、合成和抗肿瘤活性评价
甾体化合物被证明是对抗多种癌症的有效药物。肟也是经常与抗癌活性相关的化学结构。这项工作的主要目标是通过合成甾体肟并在几种癌细胞系中对其进行评估来组合这两种结构。化合物 (17 E ) -5α-androst -3-en-17-one 肟 ( 3,4 – OLOX ), (17 E )-3α,4α-epoxy-5α-androstan-17-one 肟 ( 3,4 – EPOX ), (17 E )-androst-4-en-17-one 肟 ( 4,5 – OLOX ) 和 (17 E )-4α,5α-epoxyandrostan-17-one 肟 ( 4,5 – EPOX) 被合成并在四种人类癌细胞系中评估它们的细胞毒性,即结肠直肠腺癌 (WiDr)、非小细胞肺癌 (H1299)、前列腺癌 (PC3) 和肝细胞癌 (HepG2)。还使用了人类非肿瘤细胞系 CCD841 CoN(正常结肠细胞系)。进行MTT测定、流式细胞术、荧光和血液相容性技术以进一步分析化合物的细胞毒性。3,4 – OLOX是降低所有细胞系中肿瘤细胞增殖的最有效化合物,尤其是在 WiDr (IC 50 = 9.1 μM) 和 PC3 (IC 50 = 13.8 μM) 中。4,5 – OLOX在相同的细胞系中也显示出有希望的结果( 在 WiDr 和 IC 50 中IC 50 = 16.1 μM = PC3 中的 14.5 μM)。进一步的研究还表明,3,4 – OLOX和4,5 – OLOX诱导细胞活力降低,伴随着细胞死亡的增加,主要是3,4 – OLOX在两种细胞系和4,5 中的细胞凋亡/坏死性凋亡– WiDr 细胞中的OLOX,以及4,5 – PC3 细胞中的OLOX坏死。这些化合物也可能通过产生 ROS 发挥细胞毒性,并且对非肿瘤 CCD841 CoN 细胞无毒。此外,这两种化合物都不会引起血红蛋白释放,证明静脉给药是安全的。3,4 – OLOX和4,5 – OLOX 可能是发现用于抗癌治疗的新甾体肟优化程序的起点。


























 京公网安备 11010802027423号
京公网安备 11010802027423号