当前位置:
X-MOL 学术
›
Z. Anorg. Allg. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Mn2+ substitution within the {V14As8} polyoxovanadate archetype results in {Mn2V12As8} shells with trans-positioned heterometal positions
Zeitschrift für anorganische und allgemeine Chemie ( IF 1.4 ) Pub Date : 2021-08-08 , DOI: 10.1002/zaac.202100201 Wolfgang Bensch 1 , Maren Rasmussen 2 , Christian Näther 2 , Paul Kögerler 3
Zeitschrift für anorganische und allgemeine Chemie ( IF 1.4 ) Pub Date : 2021-08-08 , DOI: 10.1002/zaac.202100201 Wolfgang Bensch 1 , Maren Rasmussen 2 , Christian Näther 2 , Paul Kögerler 3
Affiliation
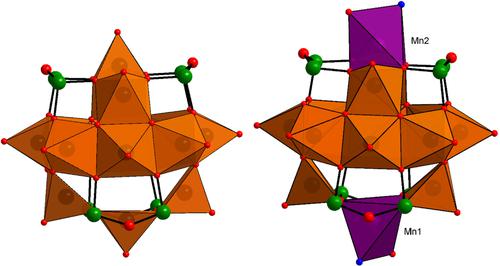
|
Solvothermal exploration of the As, V, Mn reaction system in the presence of aqueous diethylenetriamine (=dien) solution led to the discovery of [MnII4VIV12AsIII8O40(dien)4(H2O)] ⋅ 3.5H2O, containing the first arsenato-polyoxovanadate featuring a {Mn2V12As8O40} cluster shell. In comparison to its parental {V14As8O42} archetype, two vanadyl (VO2+) units at opposite ends of the polyoxovanadate shell here are substituted by Mn2+ centers. In the solid state, these {Mn2V12As8O40} units are joined into rods by sharing common Mn−O−V linkages. Expansion of the cluster shell by two further Mn2+ sites via Mn−O=V bridges generates a {Mn4V12As8O40} moiety. Three Mn2+ cations are joined by monodentate-coordinating dien ligands, which link the rods into layers. The dien ligands here coexist in three different coordination modes: monodentate, bidentate and tridentate, which is rarely observed. Magnetic susceptibility measurements show the expected significant antiferromagnetic coupling interactions between all spin centers present in the title compound, namely the isotropic spin-5/2 Mn2+ centers and the isotropic spin-1/2 vanadyl groups, in line with most other heterometal-substituted arsenato-polyoxovanadate(IV) clusters.
中文翻译:

{V14As8} 多氧钒酸盐原型内的 Mn2+ 取代导致 {Mn2V12As8} 壳具有异金属位置的转位
在二亚乙基三胺 (=dien) 水溶液存在下对 As、V、Mn 反应体系进行溶剂热探索,发现了 [Mn II 4 V IV 12 As III 8 O 40 (dien) 4 (H 2 O)] ⋅ 3.5H 2 O,包含第一个具有 {Mn 2 V 12 As 8 O 40 } 簇壳的砷酸-多氧钒酸盐。与其亲本 {V 14 As 8 O 42 } 原型相比,两个氧钒基 (VO 2+)此处聚氧钒酸盐壳相对端的单元被Mn 2+中心取代。在固态下,这些 {Mn 2 V 12 As 8 O 40 } 单元通过共享公共 Mn-O-V 键连接成棒。通过 Mn-O=V 桥由两个另外的 Mn 2+位点扩展簇壳产生 {Mn 4 V 12 As 8 O 40 } 部分。三锰2+阳离子通过单齿配位二烯配体连接,将杆连接成层。这里的二烯配体以三种不同的配位模式共存:单齿、双齿和三齿,这很少被观察到。磁化率测量表明,标题化合物中存在的所有自旋中心(即各向同性自旋 5/2 Mn 2+中心和各向同性自旋 1/2 氧钒基)之间存在预期的显着反铁磁耦合相互作用,这与大多数其他异质金属-取代的砷酸-多氧钒酸盐 (IV) 簇。
更新日期:2021-08-08
中文翻译:

{V14As8} 多氧钒酸盐原型内的 Mn2+ 取代导致 {Mn2V12As8} 壳具有异金属位置的转位
在二亚乙基三胺 (=dien) 水溶液存在下对 As、V、Mn 反应体系进行溶剂热探索,发现了 [Mn II 4 V IV 12 As III 8 O 40 (dien) 4 (H 2 O)] ⋅ 3.5H 2 O,包含第一个具有 {Mn 2 V 12 As 8 O 40 } 簇壳的砷酸-多氧钒酸盐。与其亲本 {V 14 As 8 O 42 } 原型相比,两个氧钒基 (VO 2+)此处聚氧钒酸盐壳相对端的单元被Mn 2+中心取代。在固态下,这些 {Mn 2 V 12 As 8 O 40 } 单元通过共享公共 Mn-O-V 键连接成棒。通过 Mn-O=V 桥由两个另外的 Mn 2+位点扩展簇壳产生 {Mn 4 V 12 As 8 O 40 } 部分。三锰2+阳离子通过单齿配位二烯配体连接,将杆连接成层。这里的二烯配体以三种不同的配位模式共存:单齿、双齿和三齿,这很少被观察到。磁化率测量表明,标题化合物中存在的所有自旋中心(即各向同性自旋 5/2 Mn 2+中心和各向同性自旋 1/2 氧钒基)之间存在预期的显着反铁磁耦合相互作用,这与大多数其他异质金属-取代的砷酸-多氧钒酸盐 (IV) 簇。



























 京公网安备 11010802027423号
京公网安备 11010802027423号