Journal of Structural Biology ( IF 3 ) Pub Date : 2021-08-08 , DOI: 10.1016/j.jsb.2021.107776 Subhadra Dalwani 1 , Outi Lampela 2 , Pierre Leprovost 2 , Werner Schmitz 3 , André H Juffer 2 , Rik K Wierenga 2 , Rajaram Venkatesan 1
|
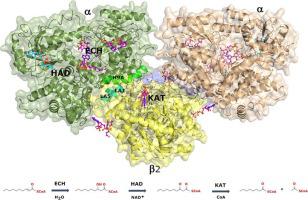
The Mycobacterium tuberculosis trifunctional enzyme (MtTFE) is an α2β2 tetrameric enzyme. The α-chain harbors the 2E-enoyl-CoA hydratase (ECH) and 3S-hydroxyacyl-CoA dehydrogenase (HAD) activities and the β-chain provides the 3-ketoacyl-CoA thiolase (KAT) activity. Enzyme kinetic data reported here show that medium and long chain enoyl-CoA molecules are preferred substrates for MtTFE. Modelling studies indicate how the linear medium and long acyl chains of these substrates can bind to each of the active sites. In addition, crystallographic binding studies have identified three new CoA binding sites which are different from the previously known CoA binding sites of the three TFE active sites. Structure comparisons provide new insights into the properties of ECH, HAD and KAT active sites of MtTFE. The interactions of the adenine moiety of CoA with loop-2 of the ECH active site cause a conformational change of this loop by which a competent ECH active site is formed. The NAD+ binding domain (domain C) of the HAD part of MtTFE has only a few interactions with the rest of the complex and adopts a range of open conformations, whereas the A-domain of the ECH part is rigidly fixed with respect to the HAD part. Two loops, the CB1-CA1 region and the catalytic CB4-CB5 loop, near the thiolase active site and the thiolase dimer interface, have high B-factors. Structure comparisons suggest that a competent and stable thiolase dimer is formed only when complexed with the α-chains, highlighting the importance of the assembly for the proper functioning of the complex.
中文翻译:

结核分枝杆菌β-氧化三功能酶的底物特异性和构象灵活性特性
结核分枝杆菌三功能酶 (MtTFE) 是一种 α 2 β 2四聚体酶。α链含有2E-烯酰-CoA 水合酶 (ECH) 和 3S-羟酰基-CoA 脱氢酶 (HAD) 活性和 β 链提供 3-酮酰基-CoA 硫解酶 (KAT) 活性。此处报道的酶动力学数据表明,中链和长链烯酰辅酶 A 分子是 MtTFE 的首选底物。建模研究表明这些底物的线性中等和长酰基链如何与每个活性位点结合。此外,晶体结合研究已经确定了三个新的 CoA 结合位点,它们不同于先前已知的三个 TFE 活性位点的 CoA 结合位点。结构比较为 MtTFE 的 ECH、HAD 和 KAT 活性位点的特性提供了新的见解。CoA 的腺嘌呤部分与 ECH 活性位点的 loop-2 的相互作用导致该环的构象变化,从而形成了有效的 ECH 活性位点。NAD+ MtTFE 的 HAD 部分的结合域(域 C)与复合物的其余部分只有少量相互作用,并采用一系列开放构象,而 ECH 部分的 A 域相对于 HAD 部分是严格固定的. 靠近硫解酶活性位点和硫解酶二聚体界面的两个环,即 CB1-CA1 区域和催化 CB4-CB5 环,具有高 B 因子。结构比较表明,只有在与 α 链复合时才能形成能胜任且稳定的硫解酶二聚体,这突出了组装对于复合物正常功能的重要性。



























 京公网安备 11010802027423号
京公网安备 11010802027423号