当前位置:
X-MOL 学术
›
ACS Infect. Dis.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
2-Mercaptomethyl Thiazolidines (MMTZs) Inhibit All Metallo-β-Lactamase Classes by Maintaining a Conserved Binding Mode
ACS Infectious Diseases ( IF 5.3 ) Pub Date : 2021-08-06 , DOI: 10.1021/acsinfecdis.1c00194 Philip Hinchliffe 1 , Diego M Moreno 2, 3 , Maria-Agustina Rossi 4 , Maria F Mojica 5, 6, 7 , Veronica Martinez 8 , Valentina Villamil 8 , Brad Spellberg 9 , George L Drusano 10 , Claudia Banchio 3, 4 , Graciela Mahler 8 , Robert A Bonomo 6, 11, 12, 13 , Alejandro J Vila 3, 4 , James Spencer 1
ACS Infectious Diseases ( IF 5.3 ) Pub Date : 2021-08-06 , DOI: 10.1021/acsinfecdis.1c00194 Philip Hinchliffe 1 , Diego M Moreno 2, 3 , Maria-Agustina Rossi 4 , Maria F Mojica 5, 6, 7 , Veronica Martinez 8 , Valentina Villamil 8 , Brad Spellberg 9 , George L Drusano 10 , Claudia Banchio 3, 4 , Graciela Mahler 8 , Robert A Bonomo 6, 11, 12, 13 , Alejandro J Vila 3, 4 , James Spencer 1
Affiliation
|
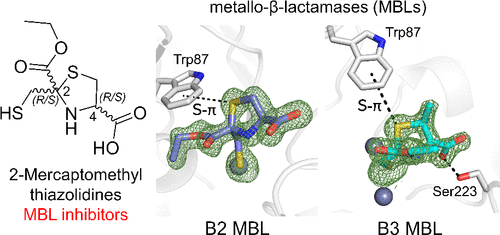
Metallo-β-lactamase (MBL) production in Gram-negative bacteria is an important contributor to β-lactam antibiotic resistance. Combining β-lactams with β-lactamase inhibitors (BLIs) is a validated route to overcoming resistance, but MBL inhibitors are not available in the clinic. On the basis of zinc utilization and sequence, MBLs are divided into three subclasses, B1, B2, and B3, whose differing active-site architectures hinder development of BLIs capable of “cross-class” MBL inhibition. We previously described 2-mercaptomethyl thiazolidines (MMTZs) as B1 MBL inhibitors (e.g., NDM-1) and here show that inhibition extends to the clinically relevant B2 (Sfh-I) and B3 (L1) enzymes. MMTZs inhibit purified MBLs in vitro (e.g., Sfh-I, Ki 0.16 μM) and potentiate β-lactam activity against producer strains. X-ray crystallography reveals that inhibition involves direct interaction of the MMTZ thiol with the mono- or dizinc centers of Sfh-I/L1, respectively. This is further enhanced by sulfur-π interactions with a conserved active site tryptophan. Computational studies reveal that the stereochemistry at chiral centers is critical, showing less potent MMTZ stereoisomers (up to 800-fold) as unable to replicate sulfur-π interactions in Sfh-I, largely through steric constraints in a compact active site. Furthermore, in silico replacement of the thiazolidine sulfur with oxygen (forming an oxazolidine) resulted in less favorable aromatic interactions with B2 MBLs, though the effect is less than that previously observed for the subclass B1 enzyme NDM-1. In the B3 enzyme L1, these effects are offset by additional MMTZ interactions with the protein main chain. MMTZs can therefore inhibit all MBL classes by maintaining conserved binding modes through different routes.
中文翻译:

2-巯基甲基噻唑烷 (MMTZ) 通过保持保守的结合模式抑制所有金属-β-内酰胺酶类别
革兰氏阴性菌中金属-β-内酰胺酶(MBL)的产生是导致β-内酰胺类抗生素耐药的重要因素。将 β-内酰胺类药物与 β-内酰胺酶抑制剂 (BLI) 联合使用是克服耐药性的有效途径,但临床上没有 MBL 抑制剂。根据锌的利用和序列,MBL 分为三个亚类,B1、B2 和 B3,其不同的活性位点结构阻碍了能够“跨类”MBL 抑制的 BLI 的发展。我们之前将 2-巯基甲基噻唑烷 (MMTZs) 描述为 B1 MBL 抑制剂(例如,NDM-1),这里表明抑制作用延伸到临床相关的 B2 (Sfh-I) 和 B3 (L1) 酶。MMTZs在体外抑制纯化的 MBLs (例如,Sfh-I、K i0.16 μM)并增强 β-内酰胺对生产菌株的活性。X 射线晶体学显示抑制涉及 MMTZ 硫醇分别与 Sfh-I/L1 的单锌或双锌中心的直接相互作用。硫-π 与保守活性位点色氨酸的相互作用进一步增强了这一点。计算研究表明,手性中心的立体化学非常关键,显示出效力较低的 MMTZ 立体异构体(高达 800 倍)无法复制 Sfh-I 中的硫-π 相互作用,主要是通过紧凑活性位点中的空间约束。此外,在 silico用氧取代噻唑烷硫(形成恶唑烷)导致与 B2 MBLs 的芳香相互作用不太有利,尽管这种影响小于先前对 B1 亚类酶 NDM-1 观察到的影响。在 B3 酶 L1 中,这些影响被额外的 MMTZ 与蛋白质主链的相互作用所抵消。因此,MMTZ 可以通过不同途径维持保守的结合模式来抑制所有 MBL 类别。
更新日期:2021-09-10
中文翻译:

2-巯基甲基噻唑烷 (MMTZ) 通过保持保守的结合模式抑制所有金属-β-内酰胺酶类别
革兰氏阴性菌中金属-β-内酰胺酶(MBL)的产生是导致β-内酰胺类抗生素耐药的重要因素。将 β-内酰胺类药物与 β-内酰胺酶抑制剂 (BLI) 联合使用是克服耐药性的有效途径,但临床上没有 MBL 抑制剂。根据锌的利用和序列,MBL 分为三个亚类,B1、B2 和 B3,其不同的活性位点结构阻碍了能够“跨类”MBL 抑制的 BLI 的发展。我们之前将 2-巯基甲基噻唑烷 (MMTZs) 描述为 B1 MBL 抑制剂(例如,NDM-1),这里表明抑制作用延伸到临床相关的 B2 (Sfh-I) 和 B3 (L1) 酶。MMTZs在体外抑制纯化的 MBLs (例如,Sfh-I、K i0.16 μM)并增强 β-内酰胺对生产菌株的活性。X 射线晶体学显示抑制涉及 MMTZ 硫醇分别与 Sfh-I/L1 的单锌或双锌中心的直接相互作用。硫-π 与保守活性位点色氨酸的相互作用进一步增强了这一点。计算研究表明,手性中心的立体化学非常关键,显示出效力较低的 MMTZ 立体异构体(高达 800 倍)无法复制 Sfh-I 中的硫-π 相互作用,主要是通过紧凑活性位点中的空间约束。此外,在 silico用氧取代噻唑烷硫(形成恶唑烷)导致与 B2 MBLs 的芳香相互作用不太有利,尽管这种影响小于先前对 B1 亚类酶 NDM-1 观察到的影响。在 B3 酶 L1 中,这些影响被额外的 MMTZ 与蛋白质主链的相互作用所抵消。因此,MMTZ 可以通过不同途径维持保守的结合模式来抑制所有 MBL 类别。



























 京公网安备 11010802027423号
京公网安备 11010802027423号