当前位置:
X-MOL 学术
›
Biotechnol. Bioeng.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Leloir glycosyltransferases enabled to flow synthesis: Continuous production of the natural C-glycoside nothofagin
Biotechnology and Bioengineering ( IF 3.8 ) Pub Date : 2021-08-06 , DOI: 10.1002/bit.27908 Hui Liu 1 , Bernd Nidetzky 1, 2
Biotechnology and Bioengineering ( IF 3.8 ) Pub Date : 2021-08-06 , DOI: 10.1002/bit.27908 Hui Liu 1 , Bernd Nidetzky 1, 2
Affiliation
|
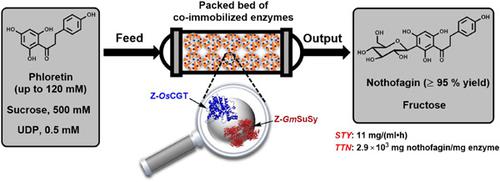
C-glycosyltransferase (CGT) and sucrose synthase (SuSy), each fused to the cationic binding module Zbasic2, were co-immobilized on anionic carrier (ReliSorb SP400) and assessed for continuous production of the natural C-glycoside nothofagin. The overall reaction was 3ʹ-C-β-glycosylation of the polyphenol phloretin from uridine 5ʹ-diphosphate (UDP)-glucose that was released in situ from sucrose and UDP. Using solid catalyst optimized for total (∼28 mg/g) as well as relative protein loading (CGT/SuSy = ∼1) and assembled into a packed bed (1 ml), we demonstrate flow synthesis of nothofagin (up to 52 mg/ml; 120 mM) from phloretin (≥95% conversion) solubilized by inclusion complexation in hydroxypropyl β-cyclodextrin. About 1.8 g nothofagin (90 ml; 12–26 mg/ml) were produced continuously over 90 reactor cycles (2.3 h/cycle) with a space-time yield of approximately 11 mg/(ml h) and a total enzyme turnover number of up to 2.9 × 103 mg/mg (=3.8 × 105 mol/mol). The co-immobilized enzymes exhibited useful effectiveness (∼40% of the enzymes in solution), with limitations on the conversion rate arising partly from external liquid–solid mass transfer of UDP under packed-bed flow conditions. The operational half-life of the catalyst (∼200 h; 30°C) was governed by the binding stability of the glycosyltransferases (≤35% loss of activity) on the solid carrier. Collectively, the current study shows integrated process technology for flow synthesis with co-immobilized sugar nucleotide-dependent glycosyltransferases, using efficient glycosylation from sucrose via the internally recycled UDP-glucose. This provides a basis from engineering science to promote glycosyltransferase applications for natural product glycosides and oligosaccharides.
中文翻译:

流式合成的 Leloir 糖基转移酶:天然 C-糖苷 nothofagin 的连续生产
C-糖基转移酶 (CGT) 和蔗糖合酶 (SuSy) 均与阳离子结合模块Z basic2 融合,共同固定在阴离子载体 (ReliSorb SP400) 上,并评估天然C-糖苷 nothofagin 的连续生产。整个反应是从蔗糖和 UDP 原位释放的尿苷 5′-二磷酸 (UDP)-葡萄糖中多酚根皮素的 3′- C - β-糖基化。使用针对总(~28 mg / g)以及相对蛋白质负载(CGT / SuSy =~1)优化的固体催化剂并组装成填充床(1 ml),我们展示了nothofagin(高达52 mg / ml; 120 mM) 来自根皮素 (≥95% 转化率) 通过包合络合溶解在羟丙基β-环糊精。在 90 个反应器循环(2.3 小时/循环)内连续产生约 1.8 g nothofagin(90 ml;12-26 mg/ml),时空产率约为 11 mg/(ml h),总酶周转数为高达 2.9 × 10 3 mg/mg (=3.8 × 10 5 摩尔/摩尔)。共固定化酶表现出有用的有效性(溶液中约 40% 的酶),转化率的限制部分是由于在填充床流动条件下 UDP 的外部液-固传质引起的。催化剂的操作半衰期(~200 小时;30°C)取决于固体载体上糖基转移酶的结合稳定性(≤35% 的活性损失)。总的来说,目前的研究显示了用于流合成与共固定的糖核苷酸依赖性糖基转移酶的集成工艺技术,使用蔗糖通过内部回收的 UDP-葡萄糖进行有效的糖基化。这为促进天然产物糖苷和寡糖的糖基转移酶应用提供了工程科学的基础。
更新日期:2021-10-13
中文翻译:

流式合成的 Leloir 糖基转移酶:天然 C-糖苷 nothofagin 的连续生产
C-糖基转移酶 (CGT) 和蔗糖合酶 (SuSy) 均与阳离子结合模块Z basic2 融合,共同固定在阴离子载体 (ReliSorb SP400) 上,并评估天然C-糖苷 nothofagin 的连续生产。整个反应是从蔗糖和 UDP 原位释放的尿苷 5′-二磷酸 (UDP)-葡萄糖中多酚根皮素的 3′- C - β-糖基化。使用针对总(~28 mg / g)以及相对蛋白质负载(CGT / SuSy =~1)优化的固体催化剂并组装成填充床(1 ml),我们展示了nothofagin(高达52 mg / ml; 120 mM) 来自根皮素 (≥95% 转化率) 通过包合络合溶解在羟丙基β-环糊精。在 90 个反应器循环(2.3 小时/循环)内连续产生约 1.8 g nothofagin(90 ml;12-26 mg/ml),时空产率约为 11 mg/(ml h),总酶周转数为高达 2.9 × 10 3 mg/mg (=3.8 × 10 5 摩尔/摩尔)。共固定化酶表现出有用的有效性(溶液中约 40% 的酶),转化率的限制部分是由于在填充床流动条件下 UDP 的外部液-固传质引起的。催化剂的操作半衰期(~200 小时;30°C)取决于固体载体上糖基转移酶的结合稳定性(≤35% 的活性损失)。总的来说,目前的研究显示了用于流合成与共固定的糖核苷酸依赖性糖基转移酶的集成工艺技术,使用蔗糖通过内部回收的 UDP-葡萄糖进行有效的糖基化。这为促进天然产物糖苷和寡糖的糖基转移酶应用提供了工程科学的基础。



























 京公网安备 11010802027423号
京公网安备 11010802027423号