当前位置:
X-MOL 学术
›
Organometallics
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
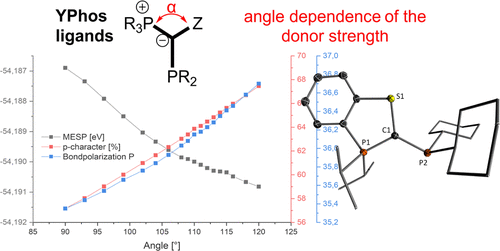
Ylide-Substituted Phosphines with a Cyclic Ylide-Backbone: Angle Dependence of the Donor Strength
Organometallics ( IF 2.8 ) Pub Date : 2021-08-04 , DOI: 10.1021/acs.organomet.1c00349 Julian Löffler 1 , Richard M Gauld 1 , Kai-Stephan Feichtner 1 , Ilja Rodstein 1 , Jana-Alina Zur 1 , Jens Handelmann 1 , Christopher Schwarz 1 , Viktoria H Gessner 1
Organometallics ( IF 2.8 ) Pub Date : 2021-08-04 , DOI: 10.1021/acs.organomet.1c00349 Julian Löffler 1 , Richard M Gauld 1 , Kai-Stephan Feichtner 1 , Ilja Rodstein 1 , Jana-Alina Zur 1 , Jens Handelmann 1 , Christopher Schwarz 1 , Viktoria H Gessner 1
Affiliation
|
Ylide-substituted phosphines (YPhos) have been shown to be highly electron-rich and efficient ligands in a variety of palladium catalyzed transformations. Here, the synthesis and characterization of novel YPhos ligands containing a cyclic backbone architecture are reported. The ligands are easily synthesized from a cyclic phosphonium salt and the chlorophosphines Cy2PCl (L1) and Cy(FluMe)PCl (L2, with FluMe = 9-methylfluorenyl) and were characterized in both solution and solid states. The smaller PCy2-substituted ligand, L1, readily formed the biscoordinate L12Pd species when treated with Pd2(dba)3 and showed no activity in palladium-catalyzed amination reactions even when applied as defined palladium(II) η3-allyl, t-Bu-indenyl, or cinnamyl precursors. Bulkier fluorenyl-substituted ligand L2 similarly was inactive, despite its ability to form the stable monophosphine complex L2·Pd(dba). Assessment of the electronic properties by experimental and computational methods revealed that L1 and L2 are considerably less electron-rich than previously synthesized YPhos ligands. This was shown to be the result of the small P–C–S bond angle, which is sterically enforced due to the cyclic nature of the backbone. Density functional theory calculations revealed that the small angle results in an increased s-character of the lone pair at the ylidic carbon atom and leads to a polarization of the C–P bond toward the carbon atom, thus decreasing the electron density at the phosphorus atom. The results demonstrate the tunability of the donor strength of YPhos ligands by modification of the ligand backbone beyond simple changes of the substitution pattern and are thus important for future ligand design, with a careful balance of many factors to be considered to achieve catalytic activity.
中文翻译:

具有环状叶立德骨架的叶立德取代膦:供体强度的角度依赖性
叶立德取代的膦 (YPhos) 已被证明在各种钯催化转化中是高度富电子和高效的配体。在这里,报道了含有环状骨架结构的新型 YPhos 配体的合成和表征。配体很容易从环状鏻盐和氯膦 Cy 2 PCl ( L1 ) 和 Cy(Flu Me )PCl ( L2,其中 Flu Me = 9-甲基芴基) 合成,并在溶液和固体状态下进行表征。当用 Pd 2处理时,较小的 PCy 2取代的配体L1很容易形成双配位L1 2 Pd物质(dba) 3并且即使在作为定义的钯 (II) η 3 -烯丙基、t -Bu-茚基或肉桂基前体使用时,在钯催化的胺化反应中也没有表现出活性。体积更大的芴基取代的配体L2同样是无活性的,尽管它能够形成稳定的单膦配合物L2 ·Pd(dba)。通过实验和计算方法对电子特性的评估表明L1和L2与以前合成的 YPhos 配体相比,电子富集程度要低得多。这被证明是 P-C-S 键角小的结果,由于骨架的环状性质,这是空间强制的。密度泛函理论计算表明,小角度导致叶立克碳原子处孤对电子的 s 特性增加,并导致 C-P 键向碳原子极化,从而降低磷原子处的电子密度. 结果证明了 YPhos 配体的供体强度的可调性,除了取代模式的简单变化之外,还可以通过修饰配体骨架来调节,因此对于未来的配体设计很重要,需要仔细平衡许多因素以实现催化活性。
更新日期:2021-08-23
中文翻译:

具有环状叶立德骨架的叶立德取代膦:供体强度的角度依赖性
叶立德取代的膦 (YPhos) 已被证明在各种钯催化转化中是高度富电子和高效的配体。在这里,报道了含有环状骨架结构的新型 YPhos 配体的合成和表征。配体很容易从环状鏻盐和氯膦 Cy 2 PCl ( L1 ) 和 Cy(Flu Me )PCl ( L2,其中 Flu Me = 9-甲基芴基) 合成,并在溶液和固体状态下进行表征。当用 Pd 2处理时,较小的 PCy 2取代的配体L1很容易形成双配位L1 2 Pd物质(dba) 3并且即使在作为定义的钯 (II) η 3 -烯丙基、t -Bu-茚基或肉桂基前体使用时,在钯催化的胺化反应中也没有表现出活性。体积更大的芴基取代的配体L2同样是无活性的,尽管它能够形成稳定的单膦配合物L2 ·Pd(dba)。通过实验和计算方法对电子特性的评估表明L1和L2与以前合成的 YPhos 配体相比,电子富集程度要低得多。这被证明是 P-C-S 键角小的结果,由于骨架的环状性质,这是空间强制的。密度泛函理论计算表明,小角度导致叶立克碳原子处孤对电子的 s 特性增加,并导致 C-P 键向碳原子极化,从而降低磷原子处的电子密度. 结果证明了 YPhos 配体的供体强度的可调性,除了取代模式的简单变化之外,还可以通过修饰配体骨架来调节,因此对于未来的配体设计很重要,需要仔细平衡许多因素以实现催化活性。



























 京公网安备 11010802027423号
京公网安备 11010802027423号