当前位置:
X-MOL 学术
›
Org. Process Res. Dev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
New Scalable Synthetic Routes to ELQ-300, ELQ-316, and Other Antiparasitic Quinolones
Organic Process Research & Development ( IF 3.4 ) Pub Date : 2021-08-04 , DOI: 10.1021/acs.oprd.1c00099 Sovitj Pou 1 , Rozalia A Dodean 1 , Lisa Frueh 1 , Katherine M Liebman 1 , Rory T Gallagher 2 , Haihong Jin 3 , Robert T Jacobs 4 , Aaron Nilsen 1, 3 , David R Stuart 2 , J Stone Doggett 1, 5 , Michael K Riscoe 1, 6 , Rolf W Winter 1
Organic Process Research & Development ( IF 3.4 ) Pub Date : 2021-08-04 , DOI: 10.1021/acs.oprd.1c00099 Sovitj Pou 1 , Rozalia A Dodean 1 , Lisa Frueh 1 , Katherine M Liebman 1 , Rory T Gallagher 2 , Haihong Jin 3 , Robert T Jacobs 4 , Aaron Nilsen 1, 3 , David R Stuart 2 , J Stone Doggett 1, 5 , Michael K Riscoe 1, 6 , Rolf W Winter 1
Affiliation
|
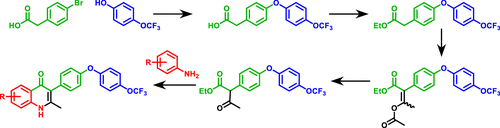
The endochin-like quinolone (ELQ) compound class may yield effective, safe treatments for a range of important human and animal afflictions. However, to access the public health potential of this compound series, a synthetic route needed to be devised, which would lower costs and be amenable to large-scale production. In the new synthetic route described here, a substituted β-keto ester, formed by an Ullmann reaction and subsequent acylation, is reacted with an aniline via a Conrad–Limpach reaction to produce 3-substituted 4(1H)-quinolones such as ELQ-300 and ELQ-316. This synthetic route, the first described to be truly amenable to industrial-scale production, is relatively short (five reaction steps), does not require palladium, chromatographic separation, or protecting group chemistry, and may be performed without high vacuum distillation.
中文翻译:

ELQ-300、ELQ-316 和其他抗寄生虫喹诺酮类药物的新可扩展合成路线
内啡肽样喹诺酮 (ELQ) 化合物类可以为一系列重要的人类和动物疾病提供有效、安全的治疗方法。然而,为了获得该化合物系列的公共卫生潜力,需要设计一种合成路线,该路线将降低成本并适合大规模生产。在这里描述的新合成路线中,通过 Ullmann 反应和随后的酰化形成取代的 β-酮酯,通过 Conrad-Limpach 反应与苯胺反应生成 3-取代的 4( 1H )-喹诺酮类,例如ELQ -300和ELQ-316. 该合成路线首次被描述为真正适合工业规模生产,相对较短(五个反应步骤),不需要钯、色谱分离或保护基化学,并且可以在没有高真空蒸馏的情况下进行。
更新日期:2021-08-20
中文翻译:

ELQ-300、ELQ-316 和其他抗寄生虫喹诺酮类药物的新可扩展合成路线
内啡肽样喹诺酮 (ELQ) 化合物类可以为一系列重要的人类和动物疾病提供有效、安全的治疗方法。然而,为了获得该化合物系列的公共卫生潜力,需要设计一种合成路线,该路线将降低成本并适合大规模生产。在这里描述的新合成路线中,通过 Ullmann 反应和随后的酰化形成取代的 β-酮酯,通过 Conrad-Limpach 反应与苯胺反应生成 3-取代的 4( 1H )-喹诺酮类,例如ELQ -300和ELQ-316. 该合成路线首次被描述为真正适合工业规模生产,相对较短(五个反应步骤),不需要钯、色谱分离或保护基化学,并且可以在没有高真空蒸馏的情况下进行。



























 京公网安备 11010802027423号
京公网安备 11010802027423号