Journal of Pharmaceutical Analysis ( IF 8.8 ) Pub Date : 2021-08-05 , DOI: 10.1016/j.jpha.2021.08.003 Yury A Gubarev 1 , Natalya Sh Lebedeva 1 , Elena S Yurina 1 , Sergey A Syrbu 1 , Aleksey N Kiselev 1, 2 , Mikhail A Lebedev 1, 2
|
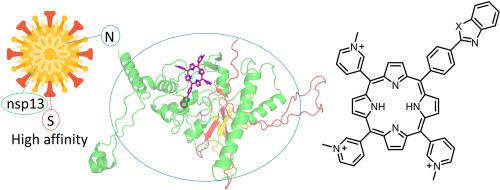
Coronavirus disease 2019 is a serious disease that causes acute respiratory syndrome and negatively affects the central nervous system. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) crosses the blood-brain barrier due to the spike (S) protein on the surface of the viral particles. Thus, it is important to develop compounds that not only have an inhibitory effect but are also capable of completely deactivating the S protein function. This study describes the purposeful modification of porphyrins and proposes compounds, asymmetrically hetaryl-substituted porphyrins with benzothiazole, benzoxazole, and N-methylbenzimidazole residues, to deactivate the S protein functions. Molecular docking of SARS-CoV-2 proteins with hetaryl-substituted porphyrins showed that the viral S protein, nucleocapsid (N) protein, and non-structural protein 13 (nsp13) exhibited the highest binding affinity.
Hetaryl-substituted porphyrins form strong complexes (13–14 kcal/mol) with the receptor-binding domain of the S protein, while the distance from the porphyrins to the receptor-binding motif (RBM) does not exceed 20 Å; therefore, RBM can be oxidized by 1O2, which is generated by porphyrin. Hetaryl-substituted porphyrins interact with the N protein in the serine/arginine-rich region, and a number of vulnerable amino acid residues are located in the photooxidation zone. This damage complicates the packaging of viral RNA into new virions. High-energy binding of hetaryl-substituted porphyrins with the N- and C-terminal domains of nsp13 was observed. This binding blocks the action of nsp13 as an enzyme of exoribonuclease and methyltransferase, thereby preventing RNA replication and processing. A procedure for the synthesis of hetaryl-substituted porphyrins was developed, new compounds were obtained, their structures were identified, and their photocatalytic properties were studied.
中文翻译:

基于不对称杂芳基取代卟啉的可能的治疗靶点和有前景的药物来对抗 SARS-CoV-2
2019 年冠状病毒病是一种严重的疾病,会导致急性呼吸系统综合症并对中枢神经系统产生负面影响。由于病毒颗粒表面的刺突 (S) 蛋白,严重急性呼吸系统综合症冠状病毒 2 (SARS-CoV-2) 可穿过血脑屏障。因此,重要的是开发不仅具有抑制作用而且能够完全失活 S 蛋白功能的化合物。本研究描述了卟啉的有目的的修饰,并提出了化合物,即具有苯并噻唑、苯并恶唑和 N-甲基苯并咪唑残基的不对称杂芳基取代的卟啉,以停用 S 蛋白功能。SARS-CoV-2 蛋白与杂芳基取代卟啉的分子对接表明,病毒 S 蛋白、核衣壳 (N) 蛋白、
杂芳基取代的卟啉与 S 蛋白的受体结合域形成强复合物 (13–14 kcal/mol),而卟啉与受体结合基序 (RBM) 的距离不超过 20 Å;因此,RBM 可以被1 O 2氧化,由卟啉产生。杂芳基取代的卟啉在富含丝氨酸/精氨酸的区域与 N 蛋白相互作用,许多易受攻击的氨基酸残基位于光氧化区。这种损伤使病毒 RNA 包装成新病毒粒子变得复杂。观察到杂芳基取代的卟啉与 nsp13 的 N 和 C 端域的高能结合。这种结合阻止了 nsp13 作为外切核糖核酸酶和甲基转移酶的酶的作用,从而阻止了 RNA 的复制和加工。开发了合成杂芳基取代的卟啉的方法,获得了新化合物,鉴定了它们的结构,并研究了它们的光催化性能。


























 京公网安备 11010802027423号
京公网安备 11010802027423号