当前位置:
X-MOL 学术
›
Chemistryopen
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Switch From Pauli-Lowering to LUMO-Lowering Catalysis in Brønsted Acid-Catalyzed Aza-Diels-Alder Reactions
ChemistryOpen ( IF 2.3 ) Pub Date : 2021-08-05 , DOI: 10.1002/open.202100172 Song Yu 1 , F Matthias Bickelhaupt 1, 2 , Trevor A Hamlin 1
ChemistryOpen ( IF 2.3 ) Pub Date : 2021-08-05 , DOI: 10.1002/open.202100172 Song Yu 1 , F Matthias Bickelhaupt 1, 2 , Trevor A Hamlin 1
Affiliation
|
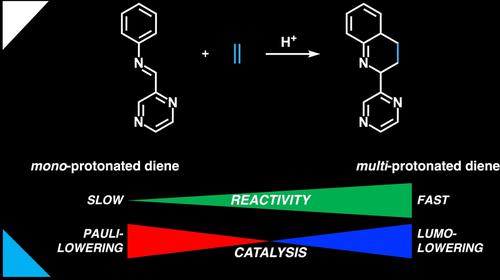
Brønsted acid-catalyzed inverse-electron demand (IED) aza-Diels-Alder reactions between 2-aza-dienes and ethylene were studied using quantum chemical calculations. The computed activation energy systematically decreases as the basic sites of the diene progressively become protonated. Our activation strain and Kohn-Sham molecular orbital analyses traced the origin of this enhanced reactivity to i) “Pauli-lowering catalysis” for mono-protonated 2-aza-dienes due to the induction of an asynchronous, but still concerted, reaction pathway that reduces the Pauli repulsion between the reactants; and ii) “LUMO-lowering catalysis” for multi-protonated 2-aza-dienes due to their highly stabilized LUMO(s) and more concerted synchronous reaction path that facilitates more efficient orbital overlaps in IED interactions. In all, we illustrate how the novel concept of “Pauli-lowering catalysis” can be overruled by the traditional concept of “LUMO-lowering catalysis” when the degree of LUMO stabilization is extreme as in the case of multi-protonated 2-aza-dienes.
中文翻译:

在布朗斯台德酸催化的氮杂-狄尔斯-阿尔德反应中从 Pauli-Lowering 切换到 LUMO-Lowering 催化
使用量子化学计算研究了 2-氮杂二烯和乙烯之间的布朗斯台德酸催化逆电子需求 (IED) 氮杂-狄尔斯-阿尔德反应。随着二烯的碱性位点逐渐质子化,计算的活化能系统地降低。我们的活化应变和 Kohn-Sham 分子轨道分析将这种增强反应性的起源追溯到 i)由于诱导异步但仍然协调的反应途径,单质子化 2-氮杂二烯的“泡利降低催化”减少反应物之间的泡利斥力;ii) “降低 LUMO 催化”-质子化的 2-氮杂二烯,因为它们具有高度稳定的 LUMO(s) 和更协调的同步反应路径,有助于 IED 相互作用中更有效的轨道重叠。总而言之,我们说明了当 LUMO 稳定化程度极端时,如多质子化 2-氮杂-二烯。
更新日期:2021-08-05
中文翻译:

在布朗斯台德酸催化的氮杂-狄尔斯-阿尔德反应中从 Pauli-Lowering 切换到 LUMO-Lowering 催化
使用量子化学计算研究了 2-氮杂二烯和乙烯之间的布朗斯台德酸催化逆电子需求 (IED) 氮杂-狄尔斯-阿尔德反应。随着二烯的碱性位点逐渐质子化,计算的活化能系统地降低。我们的活化应变和 Kohn-Sham 分子轨道分析将这种增强反应性的起源追溯到 i)由于诱导异步但仍然协调的反应途径,单质子化 2-氮杂二烯的“泡利降低催化”减少反应物之间的泡利斥力;ii) “降低 LUMO 催化”-质子化的 2-氮杂二烯,因为它们具有高度稳定的 LUMO(s) 和更协调的同步反应路径,有助于 IED 相互作用中更有效的轨道重叠。总而言之,我们说明了当 LUMO 稳定化程度极端时,如多质子化 2-氮杂-二烯。


























 京公网安备 11010802027423号
京公网安备 11010802027423号