当前位置:
X-MOL 学术
›
J. Cell. Physiol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
The protein kinase LKB1 promotes self-renewal and blocks invasiveness in glioblastoma
Journal of Cellular Physiology ( IF 5.6 ) Pub Date : 2021-08-05 , DOI: 10.1002/jcp.30542 Laia Caja 1 , Mahsa Shahidi Dadras 1, 2 , Artur Mezheyeuski 2 , Dorival Mendes Rodrigues-Junior 1 , Sijia Liu 3 , Anna Taylor Webb 1, 4 , Maria Catalina Gomez-Puerto 3 , Peter Ten Dijke 3 , Carl-Henrik Heldin 1 , Aristidis Moustakas 1
Journal of Cellular Physiology ( IF 5.6 ) Pub Date : 2021-08-05 , DOI: 10.1002/jcp.30542 Laia Caja 1 , Mahsa Shahidi Dadras 1, 2 , Artur Mezheyeuski 2 , Dorival Mendes Rodrigues-Junior 1 , Sijia Liu 3 , Anna Taylor Webb 1, 4 , Maria Catalina Gomez-Puerto 3 , Peter Ten Dijke 3 , Carl-Henrik Heldin 1 , Aristidis Moustakas 1
Affiliation
|
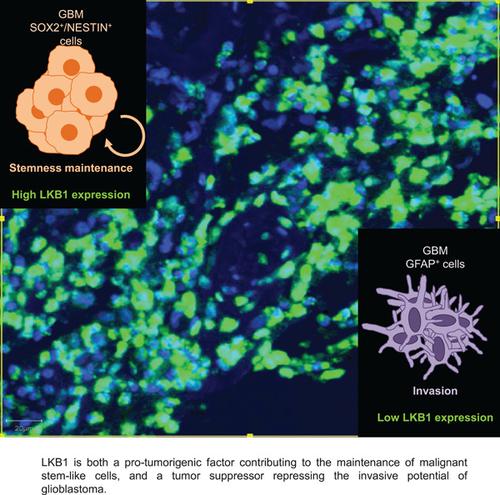
The role of liver kinase B1 (LKB1) in glioblastoma (GBM) development remains poorly understood. LKB1 may regulate GBM cell metabolism and has been suggested to promote glioma invasiveness. After analyzing LKB1 expression in GBM patient mRNA databases and in tumor tissue via multiparametric immunohistochemistry, we observed that LKB1 was localized and enriched in GBM tumor cells that co-expressed SOX2 and NESTIN stemness markers. Thus, LKB1-specific immunohistochemistry can potentially reveal subpopulations of stem-like cells, advancing GBM patient molecular pathology. We further analyzed the functions of LKB1 in patient-derived GBM cultures under defined serum-free conditions. Silencing of endogenous LKB1 impaired 3D-gliomasphere frequency and promoted GBM cell invasion in vitro and in the zebrafish collagenous tail after extravasation of circulating GBM cells. Moreover, loss of LKB1 function revealed mitochondrial dysfunction resulting in decreased ATP levels. Treatment with the clinically used drug metformin impaired 3D-gliomasphere formation and enhanced cytotoxicity induced by temozolomide, the primary chemotherapeutic drug against GBM. The IC50 of temozolomide in the GBM cultures was significantly decreased in the presence of metformin. This combinatorial effect was further enhanced after LKB1 silencing, which at least partially, was due to increased apoptosis. The expression of genes involved in the maintenance of tumor stemness, such as growth factors and their receptors, including members of the platelet-derived growth factor (PDGF) family, was suppressed after LKB1 silencing. The defect in gliomasphere growth caused by LKB1 silencing was bypassed after supplementing the cells with exogenous PFDGF-BB. Our data support the parallel roles of LKB1 in maintaining mitochondrial homeostasis, 3D-gliomasphere survival, and hindering migration in GBM. Thus, the natural loss of, or pharmacological interference with LKB1 function, may be associated with benefits in patient survival but could result in tumor spread.
中文翻译:

蛋白激酶 LKB1 促进自我更新并阻断胶质母细胞瘤的侵袭性
肝激酶 B1 (LKB1) 在胶质母细胞瘤 (GBM) 发展中的作用仍然知之甚少。LKB1 可调节 GBM 细胞代谢,并已被建议促进胶质瘤侵袭性。在通过多参数免疫组织化学分析 GBM 患者 mRNA 数据库和肿瘤组织中的 LKB1 表达后,我们观察到 LKB1 在共表达 SOX2 和 NESTIN 干性标志物的 GBM 肿瘤细胞中定位和富集。因此,LKB1 特异性免疫组织化学可以潜在地揭示干细胞样细胞的亚群,推进 GBM 患者的分子病理学。我们进一步分析了 LKB1 在确定的无血清条件下在患者来源的 GBM 培养物中的功能。内源性 LKB1 的沉默会损害 3D-gliomasphere 频率并促进 GBM 细胞在体外和循环 GBM 细胞外渗后斑马鱼胶原尾中的侵袭。此外,LKB1 功能的丧失表明线粒体功能障碍导致 ATP 水平降低。用临床使用的药物二甲双胍治疗会损害 3D-gliomasphere 的形成并增强由替莫唑胺(针对 GBM 的主要化疗药物)诱导的细胞毒性。集成电路50在二甲双胍存在下,GBM 培养物中替莫唑胺的含量显着降低。这种组合效应在 LKB1 沉默后进一步增强,这至少部分是由于细胞凋亡增加。在 LKB1 沉默后,参与维持肿瘤干性的基因的表达被抑制,例如生长因子及其受体,包括血小板衍生生长因子 (PDGF) 家族的成员。在向细胞补充外源 PFDGF-BB 后,由 LKB1 沉默引起的胶质瘤生长缺陷被绕过。我们的数据支持 LKB1 在维持线粒体稳态、3D 胶质瘤生存和阻碍 GBM 迁移方面的平行作用。因此,LKB1 功能的自然丧失或药理干扰,
更新日期:2021-08-05
中文翻译:

蛋白激酶 LKB1 促进自我更新并阻断胶质母细胞瘤的侵袭性
肝激酶 B1 (LKB1) 在胶质母细胞瘤 (GBM) 发展中的作用仍然知之甚少。LKB1 可调节 GBM 细胞代谢,并已被建议促进胶质瘤侵袭性。在通过多参数免疫组织化学分析 GBM 患者 mRNA 数据库和肿瘤组织中的 LKB1 表达后,我们观察到 LKB1 在共表达 SOX2 和 NESTIN 干性标志物的 GBM 肿瘤细胞中定位和富集。因此,LKB1 特异性免疫组织化学可以潜在地揭示干细胞样细胞的亚群,推进 GBM 患者的分子病理学。我们进一步分析了 LKB1 在确定的无血清条件下在患者来源的 GBM 培养物中的功能。内源性 LKB1 的沉默会损害 3D-gliomasphere 频率并促进 GBM 细胞在体外和循环 GBM 细胞外渗后斑马鱼胶原尾中的侵袭。此外,LKB1 功能的丧失表明线粒体功能障碍导致 ATP 水平降低。用临床使用的药物二甲双胍治疗会损害 3D-gliomasphere 的形成并增强由替莫唑胺(针对 GBM 的主要化疗药物)诱导的细胞毒性。集成电路50在二甲双胍存在下,GBM 培养物中替莫唑胺的含量显着降低。这种组合效应在 LKB1 沉默后进一步增强,这至少部分是由于细胞凋亡增加。在 LKB1 沉默后,参与维持肿瘤干性的基因的表达被抑制,例如生长因子及其受体,包括血小板衍生生长因子 (PDGF) 家族的成员。在向细胞补充外源 PFDGF-BB 后,由 LKB1 沉默引起的胶质瘤生长缺陷被绕过。我们的数据支持 LKB1 在维持线粒体稳态、3D 胶质瘤生存和阻碍 GBM 迁移方面的平行作用。因此,LKB1 功能的自然丧失或药理干扰,



























 京公网安备 11010802027423号
京公网安备 11010802027423号