Tetrahedron ( IF 2.1 ) Pub Date : 2021-08-04 , DOI: 10.1016/j.tet.2021.132374 Mohammad Bagher Teimouri 1 , Elham Batebi 1 , Shabnam Mohammadnia 1 , Hamid Reza Khavasi 2
|
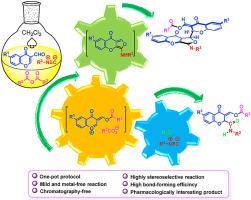
An efficient protocol for the selective synthesis of (acyloxymethylidene)chromonyl-furochromones or amido-(acyloxymethylidene)chromones via a water-controlled multicomponent cascade reaction of 3-formylchromones, linear carboxylic acid anhydrides, and alkyl isocanides in CH2Cl2 has been developed. (Acyloxymethylidene)chromonyl-furochromones were obtained in dry CH2Cl2; conversely, amido-(acyloxymethylidene)chromones were formed with wet CH2Cl2 (undried dichloromethane) as the solvent at room temperature. The reaction proceeds via the formation of a highly reactive O-acylatedoxonium intermediate, generated from the reaction between 3-formylchromones and linear carboxylic acid anhydrides. The subsequent trapping of the O-acylatedoxonium ion by aminofurochromone intermediate in situ generated by cycloaddition reaction of alkyl isocyanide and the second equivalent of 3-formylchromone in dry CH2Cl2, would give (acyloxymethylidene)chromonyl-furochromones in excellent diastereoselectivity and yields. Alternatively, O-acylatedoxonium intermediate could be also regioselectively trapped by α-addition of alkyl isocyanides in wet CH2Cl2 to provide amido-(acyloxymethylidene)chromones in good yields.
中文翻译:

多组分反应中水控制的选择性开关:(酰氧基亚甲基)色酮-呋喃色酮和酰氧基-(酰氧基亚甲基)色酮的一锅立体选择性合成
对的(acyloxymethylidene)chromonyl-furochromones或酰氨基(acyloxymethylidene)色酮的选择性合成的高效的协议经由3- formylchromones水控制的多组分级联反应,直链羧酸酐,并在CH烷基isocanides 2氯2已经开发. (酰氧基亚甲基)色酰基-呋喃色酮在干燥CH 2 Cl 2 中获得;相反,在室温下用湿CH 2 Cl 2 (未干燥二氯甲烷)作为溶剂形成酰氨基-(酰氧基亚甲基)色酮。反应通过形成高反应性的O-酰化氧鎓中间体,由 3-甲酰基色酮和线性羧酸酐反应生成。的随后的俘获ö通过aminofurochromone中间-acylatedoxonium离子原位被烷基异腈的环化加成反应和3-甲酰基色的无水CH第二等效产生2氯2,将给出在非对映选择性优良和产率(acyloxymethylidene)chromonyl-furochromones。或者,O-酰化氧鎓中间体也可以通过烷基异氰化物在湿CH 2 Cl 2 中的α-加成来区域选择性地捕获,从而以良好的产率提供酰氨基-(酰氧基亚甲基)色酮。

























 京公网安备 11010802027423号
京公网安备 11010802027423号