Tetrahedron ( IF 2.1 ) Pub Date : 2021-08-04 , DOI: 10.1016/j.tet.2021.132375 Praveen Kumar 1 , Rodney A. Fernandes 1 , Mohammad N. Ahmad 2, 3 , Sidharth Chopra 2, 3
|
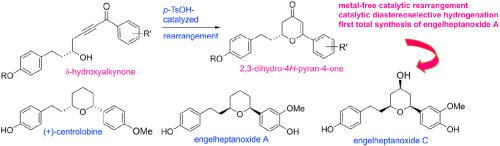
A catalytic stereoselective total synthesis of centrolobine and engelheptanoxides A and C has been completed via a metal-free catalytic δ-hydroxyalkynone rearrangement to 2,3-dihydro-4H-pyran-4-one and diastereoselective hydrogenation to the all syn-2,4,6-trisubstituted pyran strategy. The onliest required chirality was introduced by Jacobsen kinetic resolution, which further directed the diastereoselective hydrogenation. A first stereoselective synthesis of engelheptanoxide A is also accomplished. The analogues and derivatives of centrolobine and engelheptanoxides prepared were evaluated for antitubercular activity against M. tuberculosis H37Rv ATCC 27294.
中文翻译:

Centrolobine、engelheptanoxides A 和 C 及其类似物立体选择性全合成中的催化 δ-羟基炔酮重排
通过无金属催化 δ-羟基炔酮重排为 2,3-二氢-4 H-吡喃-4-one 和非对映选择性氢化为所有合成-2,完成了中心叶绿素和恩格尔庚氧化物 A 和 C 的催化立体选择性全合成, 4,6-三取代吡喃策略。唯一需要的手性是由 Jacobsen 动力学拆分引入的,这进一步指导了非对映选择性氢化。也完成了engelheptanoxide A的第一次立体选择性合成。评估了制备的中心叶酸和恩格尔庚氧化物的类似物和衍生物对结核分枝杆菌H 37 Rv ATCC 27294 的抗结核活性。


























 京公网安备 11010802027423号
京公网安备 11010802027423号