当前位置:
X-MOL 学术
›
Biochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Rimantadine Binds to and Inhibits the Influenza A M2 Proton Channel without Enantiomeric Specificity
Biochemistry ( IF 2.9 ) Pub Date : 2021-08-03 , DOI: 10.1021/acs.biochem.1c00437 Jessica L Thomaston 1 , Marley L Samways 2 , Athina Konstantinidi 3 , Chunlong Ma 4 , Yanmei Hu 4 , Hannah E Bruce Macdonald 5 , Jun Wang 4 , Jonathan W Essex 2 , William F DeGrado 1 , Antonios Kolocouris 3
Biochemistry ( IF 2.9 ) Pub Date : 2021-08-03 , DOI: 10.1021/acs.biochem.1c00437 Jessica L Thomaston 1 , Marley L Samways 2 , Athina Konstantinidi 3 , Chunlong Ma 4 , Yanmei Hu 4 , Hannah E Bruce Macdonald 5 , Jun Wang 4 , Jonathan W Essex 2 , William F DeGrado 1 , Antonios Kolocouris 3
Affiliation
|
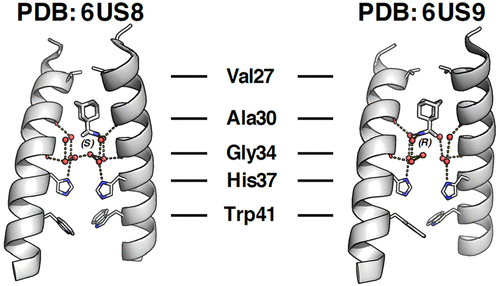
The influenza A M2 wild-type (WT) proton channel is the target of the anti-influenza drug rimantadine. Rimantadine has two enantiomers, though most investigations into drug binding and inhibition have used a racemic mixture. Solid-state NMR experiments using the full length-M2 WT have shown significant spectral differences that were interpreted to indicate tighter binding for (R)- vs (S)-rimantadine. However, it was unclear if this correlates with a functional difference in drug binding and inhibition. Using X-ray crystallography, we have determined that both (R)- and (S)-rimantadine bind to the M2 WT pore with slight differences in the hydration of each enantiomer. However, this does not result in a difference in potency or binding kinetics, as shown by similar values for kon, koff, and Kd in electrophysiological assays and for EC50 values in cellular assays. We concluded that the slight differences in hydration for the (R)- and (S)-rimantadine enantiomers are not relevant to drug binding or channel inhibition. To further explore the effect of the hydration of the M2 pore on binding affinity, the water structure was evaluated by grand canonical ensemble molecular dynamics simulations as a function of the chemical potential of the water. Initially, the two layers of ordered water molecules between the bound drug and the channel’s gating His37 residues mask the drug’s chirality. As the chemical potential becomes more unfavorable, the drug translocates down to the lower water layer, and the interaction becomes more sensitive to chirality. These studies suggest the feasibility of displacing the upper water layer and specifically recognizing the lower water layers in novel drugs.
中文翻译:

金刚乙胺结合并抑制无对映体特异性的甲型流感 M2 质子通道
甲型流感 M2 野生型 (WT) 质子通道是抗流感药物金刚乙胺的靶标。金刚乙胺有两种对映体,尽管大多数关于药物结合和抑制的研究都使用外消旋混合物。使用全长-M2 WT 的固态 NMR 实验显示出显着的光谱差异,这被解释为表明 ( R )- 与 ( S )-金刚乙胺的结合更紧密。然而,尚不清楚这是否与药物结合和抑制的功能差异相关。使用 X 射线晶体学,我们确定 ( R )- 和 ( S)-金刚乙胺与 M2 WT 孔结合,每种对映体的水合作用略有不同。然而,这不会导致效力或结合动力学的差异,如电生理测定中k on、k off和K d的相似值以及细胞测定中的 EC 50值所示。我们得出的结论是 ( R )- 和 ( S)-金刚乙胺对映体与药物结合或通道抑制无关。为了进一步探索 M2 孔的水合作用对结合亲和力的影响,通过作为水化学势函数的大正则系综分子动力学模拟来评估水结构。最初,结合药物和通道的门控 His37 残基之间的两层有序水分子掩盖了药物的手性。随着化学势变得更加不利,药物向下转移到较低的水层,并且相互作用对手性变得更加敏感。这些研究表明在新药中置换上层水层并特异性识别下层水层是可行的。
更新日期:2021-08-17
中文翻译:

金刚乙胺结合并抑制无对映体特异性的甲型流感 M2 质子通道
甲型流感 M2 野生型 (WT) 质子通道是抗流感药物金刚乙胺的靶标。金刚乙胺有两种对映体,尽管大多数关于药物结合和抑制的研究都使用外消旋混合物。使用全长-M2 WT 的固态 NMR 实验显示出显着的光谱差异,这被解释为表明 ( R )- 与 ( S )-金刚乙胺的结合更紧密。然而,尚不清楚这是否与药物结合和抑制的功能差异相关。使用 X 射线晶体学,我们确定 ( R )- 和 ( S)-金刚乙胺与 M2 WT 孔结合,每种对映体的水合作用略有不同。然而,这不会导致效力或结合动力学的差异,如电生理测定中k on、k off和K d的相似值以及细胞测定中的 EC 50值所示。我们得出的结论是 ( R )- 和 ( S)-金刚乙胺对映体与药物结合或通道抑制无关。为了进一步探索 M2 孔的水合作用对结合亲和力的影响,通过作为水化学势函数的大正则系综分子动力学模拟来评估水结构。最初,结合药物和通道的门控 His37 残基之间的两层有序水分子掩盖了药物的手性。随着化学势变得更加不利,药物向下转移到较低的水层,并且相互作用对手性变得更加敏感。这些研究表明在新药中置换上层水层并特异性识别下层水层是可行的。


























 京公网安备 11010802027423号
京公网安备 11010802027423号