Chemosphere ( IF 8.8 ) Pub Date : 2021-08-03 , DOI: 10.1016/j.chemosphere.2021.131804 Chong Wang 1 , Tianai Zhang 1 , Lifeng Yin 2 , Chengsheng Ni 1 , JiuPai Ni 1 , Li-An Hou 3
|
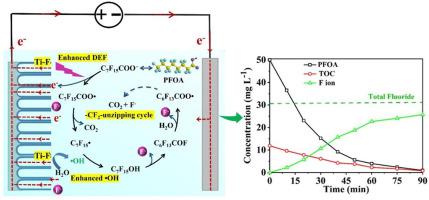
Perfluorooctanoic acid (PFOA) is of increasing concern due to its worldwide application and extremely environmental persistence. Herein, we demonstrated the electrochemical degradation of PFOA with high efficiency using the Ti3+ self-doping TiO2 nanotube arrays (Ti3+/TiO2-NTA) anode. The fabricated Ti3+/TiO2-NTA anode exhibited vertically aligned uniform nanotubes structure, and was demonstrated good performance on the electrochemical degradation of PFOA in water. The degradation rate, total organic carbon (TOC) removal rate and defluorination rate of PFOA reached 98.1 %, 93.3 % and 74.8 %, respectively, after electrolysis for 90 min at low current density of 2 mA cm−2. The energy consumption (7.6 Wh L−1) of this electrochemical oxidation system using Ti3+/TiO2-NTA anode for PFOA degradation was about 1 order of magnitude lower than using traditional PbO2 anodes. Cathodic polarization could effectively prolong the electrocatalytic activity of the anode by regenerating Ti3+ sites. PFOA molecular was underwent a rapidly mineralization to CO2 and F−, with only low concentration of short-chain perflfluorocarboxylic acids (PFCAs) intermediates identified. A possible electrochemical degradation mechanism of PFOA was proposed, in which the initial direct electron transfer (DET) on the anode to yield PFOA free radicals (C7F15COO•) and hydroxyl radicals (•OH) oxidation were greatly enhanced. This presented study provides a novel approach for the purification of the recalcitrant PFOA from wastewaters.
中文翻译:

使用 Ti3+ 自掺杂 TiO2 纳米管阵列阳极通过电化学氧化增强全氟辛酸矿化
全氟辛酸 (PFOA) 由于其全球应用和极端的环境持久性而受到越来越多的关注。在此,我们展示了使用 Ti 3+自掺杂 TiO 2纳米管阵列(Ti 3+ /TiO 2 -NTA)阳极高效电化学降解全氟辛酸。制备的 Ti 3+ /TiO 2 -NTA 阳极呈现垂直排列的均匀纳米管结构,并在水中 PFOA 的电化学降解方面表现出良好的性能。在2 mA cm -2 的低电流密度下电解90 min后,PFOA的降解率、总有机碳(TOC)去除率和脱氟率分别达到98.1 %、93.3 %和74.8 %. 这种使用Ti 3+ /TiO 2 -NTA 阳极降解PFOA 的电化学氧化系统的能耗(7.6 Wh L -1)比使用传统PbO 2阳极低约1 个数量级。阴极极化可以通过再生Ti 3+位点有效地延长阳极的电催化活性。PFOA 分子迅速矿化成 CO 2和 F -,仅鉴定出低浓度的短链全氟羧酸 (PFCA) 中间体。提出了一种可能的 PFOA 电化学降解机制,其中阳极上的初始直接电子转移(DET)产生 PFOA 自由基(C7 F 15 COO•) 和羟基自由基(•OH) 氧化得到极大增强。本研究为从废水中纯化顽固的 PFOA 提供了一种新方法。

























 京公网安备 11010802027423号
京公网安备 11010802027423号