当前位置:
X-MOL 学术
›
Environ. Sci.: Nano
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Properties and reactivity of sulfidized nanoscale zero-valent iron prepared with different borohydride amounts
Environmental Science: Nano ( IF 7.3 ) Pub Date : 2021-07-10 , DOI: 10.1039/d1en00364j Zhen Cao 1 , Hao Li 2 , Shuangyu Zhang 1 , Yunxuan Hu 1 , Jiang Xu 1 , Xinhua Xu 1
Environmental Science: Nano ( IF 7.3 ) Pub Date : 2021-07-10 , DOI: 10.1039/d1en00364j Zhen Cao 1 , Hao Li 2 , Shuangyu Zhang 1 , Yunxuan Hu 1 , Jiang Xu 1 , Xinhua Xu 1
Affiliation
|
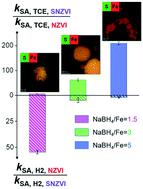
The liquid-phase reduction method with NaBH4 as the reductant is the most widely used method for sulfidized nanoscale zero-valent iron (SNZVI) synthesis. However, it is unclear how the reductant amount (e.g., NaBH4/Fe ratio) affects the physicochemical properties (i.e. Fe0 and S content, surface S speciation, and lattice constant of the Fe BCC structure) and reactivity of SNZVI. Herein, we synthesized SNZVI with different NaBH4/Fe ratios to assess how the NaBH4/Fe ratio affects their properties, and how these properties correlated to the reactivity and selectivity. The S/Fe molar ratio in the particles of SNZVI decreased from 0.15 to 0.02 when the NaBH4/Fe ratio increased from 1.5 to 5, respectively; meanwhile, the yield of SNZVI increased from 20% to 97%. The Fe0 content and the surface S2−/S22− molar ratio ([S2−/S22−]surface) increased with the increase of the NaBH4/Fe ratio, which enhanced the electron transfer of SNZVI. The trichloroethylene reactivity increased ∼8.5-fold when the [S2−/S22−]surface of SNZVI increased from 0.85 to 1.62, while the selectivity decreased from 82.8% to 64.4% accordingly, indicating that [S2−/S22−]surface could be an indicator for predicting the reactivity and selectivity of SNZVI. These results indicate that the NaBH4/Fe ratio is a possible reason for the lab-to-lab variability of SNZVI reactivity. A higher NaBH4/Fe ratio was favorable for the TCE dechlorination reaction while a lower NaBH4/Fe ratio could enhance the electron selectivity.
中文翻译:

不同硼氢化物用量制备的硫化纳米零价铁的性质和反应性
以NaBH 4为还原剂的液相还原法是目前应用最广泛的硫化纳米级零价铁(SNZVI)合成方法。然而,目前还不清楚还原剂的量(例如,NaBH 4 /Fe 比率)如何影响SNZVI的物理化学性质(即Fe 0和 S 含量、表面 S 形态和 Fe BCC 结构的晶格常数)和反应性。在此,我们合成了具有不同 NaBH 4 /Fe 比率的SNZVI,以评估 NaBH 4 /Fe 比率如何影响其性质,以及这些性质如何与反应性和选择性相关。当 NaBH 加入时,SNZVI 颗粒中的 S/Fe 摩尔比从 0.15 降低到 0.024 /Fe比分别从1.5提高到5;同时,SNZVI的产率从20%提高到97%。Fe 0含量和表面S 2- /S 2 2-摩尔比([S 2- /S 2 2- ]表面)随着NaBH 4 /Fe 比的增加而增加,这增强了SNZVI 的电子转移。当 SNZVI 的 [S 2− /S 2 2− ]表面从 0.85 增加到 1.62 时,三氯乙烯的反应性增加了~8.5 倍,而选择性相应地从 82.8% 减少到 64.4%,表明 [S 2− /S 22- ]表面可以作为预测 SNZVI 反应性和选择性的指标。这些结果表明,NaBH 4 /Fe 比率是 SNZVI 反应性的实验室间可变性的可能原因。较高的 NaBH 4 /Fe 比有利于 TCE 脱氯反应,而较低的 NaBH 4 /Fe 比可提高电子选择性。
更新日期:2021-08-03
中文翻译:

不同硼氢化物用量制备的硫化纳米零价铁的性质和反应性
以NaBH 4为还原剂的液相还原法是目前应用最广泛的硫化纳米级零价铁(SNZVI)合成方法。然而,目前还不清楚还原剂的量(例如,NaBH 4 /Fe 比率)如何影响SNZVI的物理化学性质(即Fe 0和 S 含量、表面 S 形态和 Fe BCC 结构的晶格常数)和反应性。在此,我们合成了具有不同 NaBH 4 /Fe 比率的SNZVI,以评估 NaBH 4 /Fe 比率如何影响其性质,以及这些性质如何与反应性和选择性相关。当 NaBH 加入时,SNZVI 颗粒中的 S/Fe 摩尔比从 0.15 降低到 0.024 /Fe比分别从1.5提高到5;同时,SNZVI的产率从20%提高到97%。Fe 0含量和表面S 2- /S 2 2-摩尔比([S 2- /S 2 2- ]表面)随着NaBH 4 /Fe 比的增加而增加,这增强了SNZVI 的电子转移。当 SNZVI 的 [S 2− /S 2 2− ]表面从 0.85 增加到 1.62 时,三氯乙烯的反应性增加了~8.5 倍,而选择性相应地从 82.8% 减少到 64.4%,表明 [S 2− /S 22- ]表面可以作为预测 SNZVI 反应性和选择性的指标。这些结果表明,NaBH 4 /Fe 比率是 SNZVI 反应性的实验室间可变性的可能原因。较高的 NaBH 4 /Fe 比有利于 TCE 脱氯反应,而较低的 NaBH 4 /Fe 比可提高电子选择性。



























 京公网安备 11010802027423号
京公网安备 11010802027423号