Journal of Environmental Chemical Engineering ( IF 7.7 ) Pub Date : 2021-07-30 , DOI: 10.1016/j.jece.2021.106139 Siti Zu Nurain Ahmad 1, 2 , Wan Norharyati Wan Salleh 1, 2 , Nor Hafiza Ismail 1, 2 , Nur Aqilah Mohd Razali 1, 2 , Rafidah Hamdan 3 , Ahmad Fauzi Ismail 1, 2
|
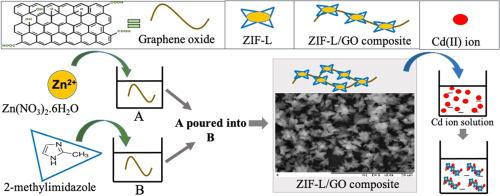
Water pollution due to heavy metal contamination has become a critical environmental issue. Thus, effective treatment for polluted water, such as simple adsorption to remove heavy metals, is gaining importance. Towards this end, zeolitic imidazolate framework-L (ZIF-L) with graphene oxide (GO) was successfully incorporated in the synthesis of novel ZIF-L/GO 20 and ZIF-L/GO 50 adsorbents using 20 and 50% loading percentages of GO, respectively, with ultrapure water as the solvent. The morphologies of ZIF-L changed to a flower-like structure with the introduction of 20% GO and turned into smaller irregular shapes when 50% GO was added. These novel adsorbents have demonstrated high adsorption capacities for Cd(II) adsorption at 172.42 mg/g (ZIF-L/GO 20) and 188.68 mg/g (ZIF-L/GO 50), with the optimum dosage of 0.2 g/L of adsorbents. The adsorption isotherms fit Langmuir isotherm, which suggested that adsorption occurred through uniform and monolayer sorption on the surface sites. The electrostatic attraction between the cationic Cd(II) and negatively charged surface played a crucial role as the main pathway in Cd(II) adsorption. Based on the investigated kinetic models, Cd(II) adsorption had a better correlation with the pseudo-second-order kinetic model, which showed that chemisorption occurred during the Cd(II) removal process. The findings of this study revealed that ZIF-L/GO has the potential of becoming the perfect adsorbent for cadmium removal from aqueous solutions.
中文翻译:

操作参数对沸石咪唑酯骨架-L/氧化石墨烯复合物处理废水除镉的影响
由于重金属污染导致的水污染已成为一个严重的环境问题。因此,对污水进行有效的处理,例如简单的吸附去除重金属,变得越来越重要。为此,沸石咪唑酯骨架-L(ZIF-L)与氧化石墨烯(GO)成功地结合到新型 ZIF-L/GO 20 和 ZIF-L/GO 50 吸附剂的合成中,使用 20% 和 50% 的负载百分比GO,分别以超纯水为溶剂。ZIF-L 的形态随着 20% GO 的引入变为花状结构,当添加 50% GO 时变成较小的不规则形状。这些新型吸附剂在 172.42 mg/g (ZIF-L/GO 20) 和 188.68 mg/g (ZIF-L/GO 50) 下表现出对 Cd(II) 的高吸附能力,最佳用量为 0.2 g/L 的吸附剂。吸附等温线符合朗缪尔等温线,这表明吸附是通过表面位点上的均匀单层吸附发生的。阳离子 Cd(II) 和带负电荷的表面之间的静电吸引力作为 Cd(II) 吸附的主要途径起着至关重要的作用。基于研究的动力学模型,Cd(II) 吸附与准二级动力学模型具有更好的相关性,这表明在 Cd(II) 去除过程中发生了化学吸附。这项研究的结果表明,ZIF-L/GO 有可能成为从水溶液中去除镉的完美吸附剂。



























 京公网安备 11010802027423号
京公网安备 11010802027423号