Critical Reviews in Oncology/Hematology ( IF 6.2 ) Pub Date : 2021-07-31 , DOI: 10.1016/j.critrevonc.2021.103434 Alessandro Inno 1 , Giandomenico Roviello 2 , Antonio Ghidini 3 , Andrea Luciani 4 , Martina Catalano 2 , Stefania Gori 1 , Fausto Petrelli 4
|
Background
The role of immune checkpoint inhibitors (ICI) rechallenge in cancer patients is not defined. When ICIs are discontinued due to treatment completion or toxicity, another course of ICIs is feasible in clinical practice, but the amount of data is still quite limited to draw definitive conclusions. Here we report the results of a meta-analysis evaluating efficacy and safety of ICI rechallenge.
Methods
PubMed, Embase, and Cochrane library were searched for studies reporting efficacy and safety of ICI rechallenge. Pooled analysis of response rate (ORR), median progression-free survival (mPFS) and median overall survival (mOS) were calculated.
Results
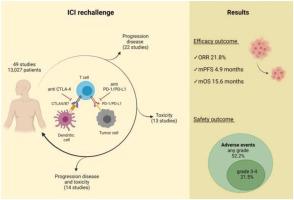
A total of 49 studies were included in qualitative and quantitative pooled analysis Overall response rate, mPFS and mOS were 21.8 % (range 0–70 %), 4.9 months (range 0–19.1 months) and 15.6 months (range 5.1–39 months), respectively. Incidence of any grade and grade 3−4 adverse events were 52.2 % (range 4–100 %) and 21.5 % (range 0–97.8 %), respectively. In the subgroup of patients who had previously discontinued ICI because of disease progression ORR, mPFS and mOS were 15.2 %, 2.9 and 7.9 months. Patients who had previously discontinued ICI because of toxicity achieved an ORR of 44 % and a mPFS of 13.2 months with the rechallenge.
Conclusions
Our results suggest that rechallenge ICI is an active and feasible strategy, and it could be considered on an individual basis. However, this analysis is based on non-randomized studies. Prospective studies are needed to clarify the role of rechallenge after disease progression or adverse events.
中文翻译:

免疫检查点抑制剂的再挑战:系统评价和荟萃分析
背景
免疫检查点抑制剂 (ICI) 再激发在癌症患者中的作用尚未确定。当因治疗完成或毒性而停用 ICIs 时,在临床实践中另一个疗程的 ICIs 是可行的,但数据量仍然相当有限,无法得出明确的结论。在这里,我们报告了评估 ICI 再挑战的有效性和安全性的荟萃分析的结果。
方法
在 PubMed、Embase 和 Cochrane 库中搜索报告 ICI 再激发的有效性和安全性的研究。计算了缓解率 (ORR)、中位无进展生存期 (mPFS) 和中位总生存期 (mOS) 的汇总分析。
结果
共有 49 项研究被纳入定性和定量汇总分析 总体反应率、mPFS 和 mOS 分别为 21.8%(范围 0-70%)、4.9 个月(范围 0-19.1 个月)和 15.6 个月(范围 5.1-39 个月) , 分别。任何级别和 3-4 级不良事件的发生率分别为 52.2%(范围 4-100%)和 21.5%(范围 0-97.8%)。在先前因疾病进展 ORR 停用 ICI 的患者亚组中,mPFS 和 mOS 分别为 15.2 %、2.9 和 7.9 个月。之前因毒性而停用 ICI 的患者在再次激发后达到了 44% 的 ORR 和 13.2 个月的 mPFS。
结论
我们的结果表明,重新挑战 ICI 是一种积极可行的策略,可以根据个人情况进行考虑。然而,该分析基于非随机研究。需要前瞻性研究来阐明疾病进展或不良事件后再次激发的作用。


























 京公网安备 11010802027423号
京公网安备 11010802027423号