当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Asymmetric Synthesis of Enantiopure Pyrrolidines by C(sp3)−H Amination of Hydrocarbons
Angewandte Chemie International Edition ( IF 16.6 ) Pub Date : 2021-07-30 , DOI: 10.1002/anie.202107898 Yanis Lazib 1 , Pascal Retailleau 2 , Tanguy Saget 3 , Benjamin Darses 4 , Philippe Dauban 5
Angewandte Chemie International Edition ( IF 16.6 ) Pub Date : 2021-07-30 , DOI: 10.1002/anie.202107898 Yanis Lazib 1 , Pascal Retailleau 2 , Tanguy Saget 3 , Benjamin Darses 4 , Philippe Dauban 5
Affiliation
|
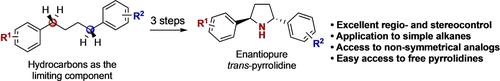
The asymmetric synthesis of enantiopure pyrrolidines is reported via a streamlined strategy relying on two sequential C−H functionalizations of simple hydrocarbons. The first step is a regio- and stereoselective catalytic nitrene C−H insertion. Then, a subsequent diastereoselective cyclization involving a 1,5-hydrogen atom transfer (HAT) from a N-centered radical leads to the formation of pyrrolidines that can then be converted to their free NH-derivatives.
中文翻译:

通过碳氢化合物的 C(sp3)-H 胺化不对称合成对映体纯吡咯烷
对映体纯吡咯烷的不对称合成是通过依赖于简单烃的两个连续 CH 官能化的简化策略报道的。第一步是区域选择性和立体选择性催化氮烯 CH 插入。然后,随后的非对映选择性环化反应涉及来自 N 中心自由基的 1,5-氢原子转移 (HAT),导致形成吡咯烷,然后可以将其转化为游离的 NH 衍生物。
更新日期:2021-09-20
中文翻译:

通过碳氢化合物的 C(sp3)-H 胺化不对称合成对映体纯吡咯烷
对映体纯吡咯烷的不对称合成是通过依赖于简单烃的两个连续 CH 官能化的简化策略报道的。第一步是区域选择性和立体选择性催化氮烯 CH 插入。然后,随后的非对映选择性环化反应涉及来自 N 中心自由基的 1,5-氢原子转移 (HAT),导致形成吡咯烷,然后可以将其转化为游离的 NH 衍生物。


























 京公网安备 11010802027423号
京公网安备 11010802027423号