当前位置:
X-MOL 学术
›
Chem. Bio. Drug Des.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Catalytic mechanism of butane anaerobic oxidation for alkyl-coenzyme M reductase
Chemical Biology & Drug Design ( IF 3 ) Pub Date : 2021-07-30 , DOI: 10.1111/cbdd.13931 Xiaopian Tian 1 , Hao Liu 2 , Hai-Feng Chen 1, 3
Chemical Biology & Drug Design ( IF 3 ) Pub Date : 2021-07-30 , DOI: 10.1111/cbdd.13931 Xiaopian Tian 1 , Hao Liu 2 , Hai-Feng Chen 1, 3
Affiliation
|
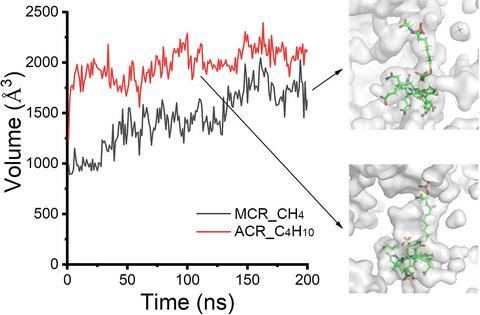
Methane is among the most potent of the greenhouse gases, which plays a key role in global climate change. As an excellent carbon and energy source, methane can be utilized by anaerobic methane oxidizing archaea and aerobic methane oxidizing bacteria. The previous work shows that an anaerobic thermophilic enrichment culture composed of dense consortia of archaea and bacteria apparently uses partly similar pathways to oxidize the C4 hydrocarbon butane. However, the catalytic mechanism of butane anaerobic oxidation for alkyl-coenzyme M reductase is still unknown. Therefore, molecular dynamics (MD) simulation was used to investigate the dynamics differences of catalytic mechanism between methane coenzyme M reductase (MCR) and alkyl-coenzyme M reductase (ACR). At first, the binding pocket of ACR is larger than that of MCR. Then, the complex of butane and ACR is more stable than that of methane and ACR. Protein conformation cloud suggests that the position of methane is dynamics and methane escapes from the binding pocket of ACR during most of the simulation time, while butane tightly binds in the pocket of ACR. The hydrophobic interactions between butane and ACR are more and stronger than those between methane and ACR. At the same time, the binding free energy between butane and ACR is significantly lower than that between methane and ACR. The dynamics correlation network indicates that the transformation of information flow for ACR-butane is smoother than that for ACR-methane. The shortest pathway for ACR-butane is from Gln144, Ala141, Hie135, Ile133, Ala160, Arg206, Asp97, Met94, Tyr347 to Phe345 with synergistic effect for two butane molecules. This study can insight into the catalytic mechanism for butane/ACR complex.
中文翻译:

烷基辅酶M还原酶丁烷厌氧氧化的催化机理
甲烷是最有效的温室气体之一,它在全球气候变化中发挥着关键作用。甲烷作为一种优良的碳源和能源,可被厌氧甲烷氧化古菌和好氧甲烷氧化细菌利用。先前的工作表明,由密集的古细菌和细菌聚生体组成的厌氧嗜热富集培养物显然使用部分相似的途径来氧化 C4 碳氢化合物丁烷。然而,丁烷厌氧氧化对烷基辅酶M还原酶的催化机制仍不清楚。因此,分子动力学(MD)模拟被用来研究甲烷辅酶M还原酶(MCR)和烷基辅酶M还原酶(ACR)之间催化机制的动力学差异。起初,ACR 的绑定口袋比 MCR 的大。然后,丁烷和 ACR 的络合物比甲烷和 ACR 的络合物更稳定。蛋白质构象云表明甲烷的位置是动态的,甲烷在大部分模拟时间内从 ACR 的结合袋中逸出,而丁烷则紧紧地结合在 ACR 的袋中。丁烷和ACR之间的疏水作用比甲烷和ACR之间的疏水作用更强。同时,丁烷与ACR之间的结合自由能明显低于甲烷与ACR之间的结合自由能。动力学相关网络表明,ACR-丁烷的信息流转换比ACR-甲烷更平滑。ACR-丁烷的最短途径是从 Gln144、Ala141、Hie135、Ile133、Ala160、Arg206、Asp97、Met94、Tyr347 到 Phe345,对两个丁烷分子具有协同作用。
更新日期:2021-07-30
中文翻译:

烷基辅酶M还原酶丁烷厌氧氧化的催化机理
甲烷是最有效的温室气体之一,它在全球气候变化中发挥着关键作用。甲烷作为一种优良的碳源和能源,可被厌氧甲烷氧化古菌和好氧甲烷氧化细菌利用。先前的工作表明,由密集的古细菌和细菌聚生体组成的厌氧嗜热富集培养物显然使用部分相似的途径来氧化 C4 碳氢化合物丁烷。然而,丁烷厌氧氧化对烷基辅酶M还原酶的催化机制仍不清楚。因此,分子动力学(MD)模拟被用来研究甲烷辅酶M还原酶(MCR)和烷基辅酶M还原酶(ACR)之间催化机制的动力学差异。起初,ACR 的绑定口袋比 MCR 的大。然后,丁烷和 ACR 的络合物比甲烷和 ACR 的络合物更稳定。蛋白质构象云表明甲烷的位置是动态的,甲烷在大部分模拟时间内从 ACR 的结合袋中逸出,而丁烷则紧紧地结合在 ACR 的袋中。丁烷和ACR之间的疏水作用比甲烷和ACR之间的疏水作用更强。同时,丁烷与ACR之间的结合自由能明显低于甲烷与ACR之间的结合自由能。动力学相关网络表明,ACR-丁烷的信息流转换比ACR-甲烷更平滑。ACR-丁烷的最短途径是从 Gln144、Ala141、Hie135、Ile133、Ala160、Arg206、Asp97、Met94、Tyr347 到 Phe345,对两个丁烷分子具有协同作用。



























 京公网安备 11010802027423号
京公网安备 11010802027423号