当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
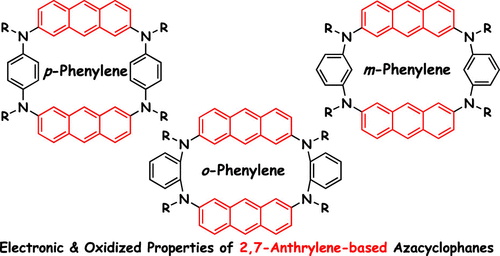
Synthesis, Structures, and Electronic Properties of 2,7-Anthrylene-Based Azacyclophanes Bearing o-, m-, and p-Phenylenediamine Linkers
The Journal of Organic Chemistry ( IF 3.6 ) Pub Date : 2021-07-29 , DOI: 10.1021/acs.joc.1c00856 Tetsuo Iwanaga 1 , Takashi Komori 1 , Hiroki Sato 1 , Shuichi Suzuki 2 , Tomokazu Yamauchi 3 , Yohji Misaki 3 , Hiroyasu Sato 4 , Shinji Toyota 5
The Journal of Organic Chemistry ( IF 3.6 ) Pub Date : 2021-07-29 , DOI: 10.1021/acs.joc.1c00856 Tetsuo Iwanaga 1 , Takashi Komori 1 , Hiroki Sato 1 , Shuichi Suzuki 2 , Tomokazu Yamauchi 3 , Yohji Misaki 3 , Hiroyasu Sato 4 , Shinji Toyota 5
Affiliation
|
A series of novel azacyclophanes consisting of 2,7-anthrylene and phenylene units were designed and synthesized by the Buchwald–Hartwig coupling reaction to investigate their unique electronic properties in multiple oxidized states. Cyclic voltammetry showed that the p-phenylene derivative exhibited three reversible oxidation waves, whereas the o- and m-phenylene derivatives showed two quasi-reversible oxidation waves due to the complicated intramolecular interaction between the oxidized units and neutral units. Moreover, the absorption spectra of the p-phenylene derivative in different oxidation states showed absorption bands at 865 and 1025 nm, which were attributed to intramolecular charge–transfer interactions. The photophysical and electrochemical properties of the p-phenylene analog were also compared with those of the o- and m-phenylene derivatives based on theoretical calculations for further evaluation of the intramolecular electronic interactions.
中文翻译:

带有邻、间和对苯二胺连接体的 2,7-蒽基氮杂环芳烃的合成、结构和电子特性
通过 Buchwald-Hartwig 偶联反应设计并合成了一系列由 2,7-蒽和亚苯基单元组成的新型氮杂环芳烃,以研究它们在多种氧化态下的独特电子性质。循环伏安法表明,p亚苯基衍生物表现出3个可逆的氧化波,而ø -和中号亚苯基衍生物表现出两个准可逆的氧化波是由于氧化单元和中性单元之间的复杂的分子内相互作用。此外,p的吸收光谱不同氧化态的-亚苯基衍生物在 865 和 1025 nm 处显示吸收带,这归因于分子内电荷转移相互作用。所述的光物理和电化学性能p亚苯基类似物也与那些相比ø -和中号基于针对所述分子内电子的相互作用的进一步评估理论计算亚苯基衍生物。
更新日期:2021-09-03
中文翻译:

带有邻、间和对苯二胺连接体的 2,7-蒽基氮杂环芳烃的合成、结构和电子特性
通过 Buchwald-Hartwig 偶联反应设计并合成了一系列由 2,7-蒽和亚苯基单元组成的新型氮杂环芳烃,以研究它们在多种氧化态下的独特电子性质。循环伏安法表明,p亚苯基衍生物表现出3个可逆的氧化波,而ø -和中号亚苯基衍生物表现出两个准可逆的氧化波是由于氧化单元和中性单元之间的复杂的分子内相互作用。此外,p的吸收光谱不同氧化态的-亚苯基衍生物在 865 和 1025 nm 处显示吸收带,这归因于分子内电荷转移相互作用。所述的光物理和电化学性能p亚苯基类似物也与那些相比ø -和中号基于针对所述分子内电子的相互作用的进一步评估理论计算亚苯基衍生物。


























 京公网安备 11010802027423号
京公网安备 11010802027423号