当前位置:
X-MOL 学术
›
RSC Chem. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
A cell permeable bimane-constrained PCNA-interacting peptide
RSC Chemical Biology Pub Date : 2021-07-21 , DOI: 10.1039/d1cb00113b Aimee J Horsfall 1, 2, 3 , Beth A Vandborg 1, 4 , Zoya Kikhtyak 5 , Denis B Scanlon 1, 2 , Wayne D Tilley 5 , Theresa E Hickey 5 , John B Bruning 1, 4 , Andrew D Abell 1, 2, 3
RSC Chemical Biology Pub Date : 2021-07-21 , DOI: 10.1039/d1cb00113b Aimee J Horsfall 1, 2, 3 , Beth A Vandborg 1, 4 , Zoya Kikhtyak 5 , Denis B Scanlon 1, 2 , Wayne D Tilley 5 , Theresa E Hickey 5 , John B Bruning 1, 4 , Andrew D Abell 1, 2, 3
Affiliation
|
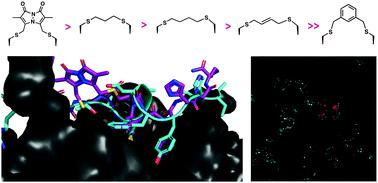
The human sliding clamp protein known as proliferating cell nuclear antigen (PCNA) orchestrates DNA-replication and -repair and as such is an ideal therapeutic target for proliferative diseases, including cancer. Peptides derived from the human p21 protein bind PCNA with high affinity via a 310-helical binding conformation and are known to shut down DNA-replication. Here, we present studies on short analogues of p21 peptides (143–151) conformationally constrained with a covalent linker between i, i + 4 separated cysteine residues at positions 145 and 149 to access peptidomimetics that target PCNA. The resulting macrocycles bind PCNA with KD values ranging from 570 nM to 3.86 μM, with the bimane-constrained peptide 7 proving the most potent. Subsequent X-ray crystallography and computational modelling studies of the macrocyclic peptides bound to PCNA indicated only the high-affinity peptide 7 adopted the classical 310-helical binding conformation. This suggests the 310-helical conformation is critical to high affinity PCNA binding, however NMR secondary shift analysis of peptide 7 revealed this secondary structure was not well-defined in solution. Peptide 7 is cell permeable and localised to the cell cytosol of breast cancer cells (MDA-MB-468), revealed by confocal microscopy showing blue fluorescence of the bimane linker. The inherent fluorescence of the bimane moiety present in peptide 7 allowed it to be directly imaged in the cell uptake assay, without attachment of an auxiliary fluorescent tag. This highlights a significant benefit of using a bimane constraint to access conformationally constrained macrocyclic peptides. This study identifies a small peptidomimetic that binds PCNA with higher affinity than previous reported p21 macrocycles, and is cell permeable, providing a significant advance toward development of a PCNA inhibitor for therapeutic applications.
中文翻译:

细胞渗透性双烷限制的 PCNA 相互作用肽
被称为增殖细胞核抗原 (PCNA) 的人类滑动钳蛋白协调 DNA 复制和修复,因此是增殖性疾病(包括癌症)的理想治疗靶点。源自人 p21 蛋白的肽通过3 10螺旋结合构象以高亲和力结合 PCNA,并且已知会关闭 DNA 复制。在这里,我们介绍了 p21 肽 (143-151) 的短类似物的研究,这些类似物在i、i + 4 个位置 145 和 149 处分离的半胱氨酸残基之间有一个共价接头,以获取靶向 PCNA 的肽模拟物。所得大环将 PCNA 与K D结合值范围从 570 nM 到 3.86 μM,其中受 bimane 约束的肽7 被证明是最有效的。随后对与 PCNA 结合的大环肽进行的 X 射线晶体学和计算建模研究表明,只有高亲和力肽7采用了经典的 3 10螺旋结合构象。这表明 3 10 -螺旋构象对于高亲和力 PCNA 结合至关重要,但是肽7 的NMR 二级位移分析显示该二级结构在溶液中没有明确定义。肽7是细胞可渗透的并定位于乳腺癌细胞 (MDA-MB-468) 的细胞质,通过共聚焦显微镜显示,显示 bimane 接头的蓝色荧光。存在于肽7中的 bimane 部分的固有荧光使其能够在细胞摄取测定中直接成像,无需附加辅助荧光标签。这突出了使用 bimane 约束访问构象受限的大环肽的显着优势。这项研究确定了一种小肽模拟物,它以比之前报道的 p21 大环化合物更高的亲和力结合 PCNA,并且具有细胞渗透性,为开发用于治疗应用的 PCNA 抑制剂提供了重大进展。
更新日期:2021-07-29
中文翻译:

细胞渗透性双烷限制的 PCNA 相互作用肽
被称为增殖细胞核抗原 (PCNA) 的人类滑动钳蛋白协调 DNA 复制和修复,因此是增殖性疾病(包括癌症)的理想治疗靶点。源自人 p21 蛋白的肽通过3 10螺旋结合构象以高亲和力结合 PCNA,并且已知会关闭 DNA 复制。在这里,我们介绍了 p21 肽 (143-151) 的短类似物的研究,这些类似物在i、i + 4 个位置 145 和 149 处分离的半胱氨酸残基之间有一个共价接头,以获取靶向 PCNA 的肽模拟物。所得大环将 PCNA 与K D结合值范围从 570 nM 到 3.86 μM,其中受 bimane 约束的肽7 被证明是最有效的。随后对与 PCNA 结合的大环肽进行的 X 射线晶体学和计算建模研究表明,只有高亲和力肽7采用了经典的 3 10螺旋结合构象。这表明 3 10 -螺旋构象对于高亲和力 PCNA 结合至关重要,但是肽7 的NMR 二级位移分析显示该二级结构在溶液中没有明确定义。肽7是细胞可渗透的并定位于乳腺癌细胞 (MDA-MB-468) 的细胞质,通过共聚焦显微镜显示,显示 bimane 接头的蓝色荧光。存在于肽7中的 bimane 部分的固有荧光使其能够在细胞摄取测定中直接成像,无需附加辅助荧光标签。这突出了使用 bimane 约束访问构象受限的大环肽的显着优势。这项研究确定了一种小肽模拟物,它以比之前报道的 p21 大环化合物更高的亲和力结合 PCNA,并且具有细胞渗透性,为开发用于治疗应用的 PCNA 抑制剂提供了重大进展。


























 京公网安备 11010802027423号
京公网安备 11010802027423号