Journal of Hepatology ( IF 25.7 ) Pub Date : 2021-07-29 , DOI: 10.1016/j.jhep.2021.07.021 Jessie Torgersen 1 , Craig W Newcomb 2 , Dena M Carbonari 2 , Christopher T Rentsch 3 , Lesley S Park 4 , Alyssa Mezochow 5 , Rajni L Mehta 6 , Lynn Buchwalder 6 , Janet P Tate 6 , Norbert Bräu 7 , Debika Bhattacharya 8 , Joseph K Lim 6 , Tamar H Taddei 6 , Amy C Justice 9 , Vincent Lo Re 1
|
Background & Aims
Cases of acute liver injury (ALI) have been reported among chronic HCV-infected patients receiving protease inhibitor (PI)-based direct-acting antiviral (DAA) regimens, but no analyses have compared the risk of ALI in patients receiving PI- vs. non-PI-based DAAs. Thus, we compared the risk of 3 ALI outcomes between patients (by baseline Fibrosis-4 [FIB-4] group) receiving PI-based or non-PI-based DAAs.
Methods
We conducted a cohort study of 18,498 patients receiving PI-based DAA therapy (paritaprevir/ritonavir/ombitasvir±dasabuvir, elbasvir/grazoprevir, glecaprevir/pibrentasvir) matched 1:1 on propensity score to those receiving non-PI-based DAAs (sofosbuvir/ledipasvir, sofosbuvir/velpatasvir) in the 1945-1965 Veterans Birth Cohort (2014-2019). During exposure to DAA therapy, we determined development of: i) alanine aminotransferase (ALT) >200 U/L, ii) severe hepatic dysfunction (coagulopathy with hyperbilirubinemia), and iii) hepatic decompensation. We used Cox regression to determine hazard ratios (HRs) with 95% CIs for each ALI outcome within groups defined by baseline FIB-4 (≤3.25; >3.25).
Results
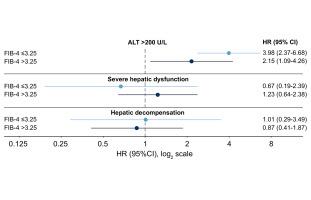
Among patients with baseline FIB-4 ≤3.25, those receiving PIs had a higher risk of ALT >200 U/L (HR 3.98; 95% CI 2.37-6.68), but not severe hepatic dysfunction (HR 0.67; 95% CI 0.19-2.39) or hepatic decompensation (HR 1.01; 95% CI 0.29-3.49), compared to those receiving non-PI-based regimens. For those with baseline FIB-4 >3.25, those receiving PIs had a higher risk of ALT >200 U/L (HR, 2.15; 95% CI 1.09-4.26), but not severe hepatic dysfunction (HR, 1.23 [0.64-2.38]) or hepatic decompensation (HR, 0.87; 95% CI 0.41-1.87), compared to those receiving non-PI-based regimens
Conclusion
While risk of incident ALT elevations was increased in those receiving PI-based DAAs in both FIB-4 groups, the risk of severe hepatic dysfunction and hepatic decompensation did not differ between patients receiving PI- or non-PI-based DAAs in either FIB-4 group.
Lay summary
Cases of liver injury have been reported among patients treated with protease inhibitor-based direct-acting antivirals for hepatitis C infection, but it is not clear if the risk of liver injury among people starting these drugs is increased compared to those starting non-protease inhibitor-based therapy. In this study, patients receiving protease inhibitor-based treatment had a higher risk of liver inflammation than those receiving a non-protease inhibitor-based treatment, regardless of the presence of pre-treatment advanced liver fibrosis/cirrhosis. However, the risk of severe liver dysfunction and decompensation were not higher for patients treated with protease inhibitor-based regimens.
中文翻译:

基于蛋白酶抑制剂的直接抗病毒药物与转氨酶升高的风险增加有关,但与肝功能障碍或失代偿无关
背景与目标
在接受基于蛋白酶抑制剂 (PI) 的直接作用抗病毒 (DAA) 方案的慢性 HCV 感染患者中,已有急性肝损伤 (ALI) 病例的报道,但尚无分析比较接受 PI-与非直接作用抗病毒 (DAA) 方案的患者发生 ALI 的风险。非基于 PI 的 DAA。因此,我们比较了接受基于 PI 或非基于 PI 的 DAA 的患者(按基线 Fibrosis-4 [FIB-4] 组)发生 3 种 ALI 结果的风险。
方法
我们对接受基于 PI 的 DAA 治疗(paritaprevir/ritonavir/ombitasvir±dasabuvir、elbasvir/grazoprevir、glecaprevir/pibrentasvir)的 18,498 名患者进行了队列研究,其倾向评分与接受非基于 PI 的 DAA 治疗(sofosbuvir/ ledipasvir、sofosbuvir/velpatasvir)在 1945-1965 年退伍军人出生队列(2014-2019)中。在接受 DAA 治疗期间,我们确定了以下情况的发展:i) 丙氨酸转氨酶 (ALT) >200 U/L,ii) 严重的肝功能障碍(凝血病伴高胆红素血症),以及 iii) 肝功能失代偿。我们使用 Cox 回归来确定由基线 FIB-4(≤3.25;>3.25)定义的组内每个 ALI 结果的风险比 (HR) 和 95% CI。
结果
在基线 FIB-4 ≤3.25 的患者中,接受 PI 的患者 ALT >200 U/L 的风险较高(HR 3.98;95% CI 2.37-6.68),但不存在严重肝功能障碍(HR 0.67;95% CI 0.19- 2.39) 或肝功能失代偿(HR 1.01;95% CI 0.29-3.49),与接受非 PI 方案的患者相比。对于基线 FIB-4 >3.25 的患者,接受 PI 的患者 ALT >200 U/L 的风险更高(HR,2.15;95% CI 1.09-4.26),但不存在严重肝功能障碍(HR,1.23 [0.64-2.38) ]) 或肝功能失代偿(HR,0.87;95% CI 0.41-1.87),与接受非 PI 方案的患者相比
结论
虽然在两个 FIB-4 组中接受基于 PI 的 DAA 的患者发生 ALT 升高的风险增加,但在 FIB-4 组中接受基于 PI 或非基于 PI 的 DAA 的患者之间严重肝功能障碍和肝失代偿的风险没有差异4组。
外行总结
在接受基于蛋白酶抑制剂的直接抗病毒药物治疗丙型肝炎感染的患者中,已有肝损伤病例报告,但尚不清楚与开始使用非蛋白酶抑制剂的患者相比,开始使用这些药物的患者发生肝损伤的风险是否增加为基础的治疗。在这项研究中,接受基于蛋白酶抑制剂治疗的患者比接受基于非蛋白酶抑制剂治疗的患者有更高的肝脏炎症风险,无论治疗前是否存在晚期肝纤维化/肝硬化。然而,接受基于蛋白酶抑制剂的方案治疗的患者出现严重肝功能障碍和失代偿的风险并不高。



























 京公网安备 11010802027423号
京公网安备 11010802027423号