当前位置:
X-MOL 学术
›
J. Cell. Biochem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
A new functional role of mitochondria-anchored protein ligase in peroxisome morphology in mammalian cells
Journal of Cellular Biochemistry ( IF 4 ) Pub Date : 2021-07-28 , DOI: 10.1002/jcb.30114 Abhishek Mohanty 1, 2 , Rodolfo Zunino 3 , Vincent Soubannier 3 , Shilpa Dilipkumar 4
Journal of Cellular Biochemistry ( IF 4 ) Pub Date : 2021-07-28 , DOI: 10.1002/jcb.30114 Abhishek Mohanty 1, 2 , Rodolfo Zunino 3 , Vincent Soubannier 3 , Shilpa Dilipkumar 4
Affiliation
|
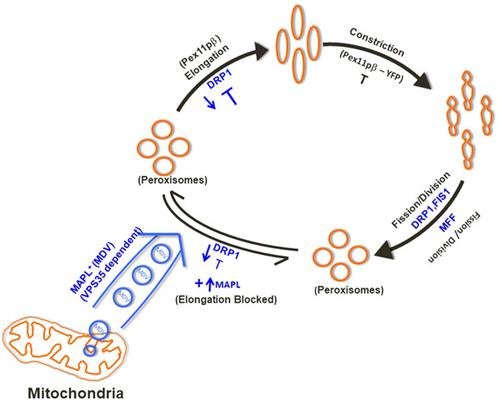
Mitochondria and peroxisomes are metabolically interconnected and functionally active subcellular organelles. These two dynamic organelles, share a number of common biochemical functions such as β-oxidation of fatty acids and detoxification of peroxides. The biogenesis and morphology of both these organelles in the mammalian cells is controlled by common transcription factors like PGC1α, and by a common fission machinery comprising of fission proteins like DRP1, Mff, and hFis1, respectively. In addition, the outer membrane mitochondria-anchored protein ligase (MAPL), the first mitochondrial SUMO E3 ligase with a RING-finger domain, also regulates mitochondrial morphology inducing mitochondrial fragmentation upon its overexpression. This fragmentation is dependent on both the RING domain of MAPL and the presence of the mitochondrial fission GTPase dynamin-related protein-1 (DRP1). Earlier studies have demonstrated that mitochondrial-derived vesicles are formed independently of the known mitochondrial fission GTPase, DRP1 are enriched for MAPL and are targeted to peroxisomes. The current study shows that MAPL regulates morphology of peroxisomes in a cell-type specific manner. Fascinatingly, the peroxisome elongation caused either due to silencing of DRP1 or by addition of polyunsaturated fatty acid, docosahexaenoic acid was blocked by overexpressing MAPL in mammalian cell lines. Furthermore, the transfection and colocalisation studies of MAPL with peroxisome membrane marker, PMP70, in different cell lines clearly revealed a cell-type specificity of transport of MAPL to peroxisomes. Previous work has placed the Vps35 (retromer component) as vital for delivery of MAPL to peroxisomes, placing the retromer as critical for the formation of MAPL-positive mitochondrial-derived vesicles. The results of polyethylene glycol-based cell–cell fusion assay signified that the enrichment of MAPL in peroxisomes is through vesicles and a retromer dependent phenomenon. Thus, a novel function for MAPL in peroxisomes is established to regulate peroxisome elongation and morphology under growth conditions and thus possibly modulate peroxisome fission.
中文翻译:

线粒体锚定蛋白连接酶在哺乳动物细胞过氧化物酶体形态中的新功能作用
线粒体和过氧化物酶体是代谢相互关联且功能活跃的亚细胞器。这两个动态细胞器具有许多共同的生化功能,例如脂肪酸的β-氧化和过氧化物的解毒。哺乳动物细胞中这两种细胞器的生物发生和形态均由常见的转录因子(如 PGC1α)和由裂变蛋白(如 DRP1、Mff 和 hFis1)组成的常见裂变机制控制。此外,外膜线粒体锚定蛋白连接酶 (MAPL) 是第一个具有环指结构域的线粒体 SUMO E3 连接酶,它也调节线粒体形态,在其过表达时诱导线粒体断裂。这种片段化取决于 MAPL 的 RING 结构域和线粒体裂变 GTPase dynamin-related protein-1 (DRP1) 的存在。早期的研究表明,线粒体衍生的囊泡是独立于已知的线粒体裂变 GTPase 形成的,DRP1 富含 MAPL 并靶向过氧化物酶体。目前的研究表明,MAPL 以细胞类型特异性方式调节过氧化物酶体的形态。令人着迷的是,由于 DRP1 的沉默或通过添加多不饱和脂肪酸引起的过氧化物酶体延长,二十二碳六烯酸被哺乳动物细胞系中过表达的 MAPL 所阻断。此外,MAPL 与过氧化物酶体膜标记物 PMP70 的转染和共定位研究,在不同的细胞系中清楚地揭示了 MAPL 向过氧化物酶体转运的细胞类型特异性。以前的工作已将 Vps35(逆转录酶组分)作为将 MAPL 递送至过氧化物酶体的关键,将逆转录酶作为形成 MAPL 阳性线粒体衍生囊泡的关键。基于聚乙二醇的细胞-细胞融合试验结果表明,MAPL 在过氧化物酶体中的富集是通过囊泡和逆转录酶依赖性现象。因此,建立了 MAPL 在过氧化物酶体中的新功能,以在生长条件下调节过氧化物酶体的伸长和形态,从而可能调节过氧化物酶体的裂变。将逆转录酶作为MAPL阳性线粒体衍生囊泡形成的关键。基于聚乙二醇的细胞-细胞融合试验结果表明,MAPL 在过氧化物酶体中的富集是通过囊泡和逆转录酶依赖性现象。因此,建立了 MAPL 在过氧化物酶体中的新功能,以在生长条件下调节过氧化物酶体的伸长和形态,从而可能调节过氧化物酶体的裂变。将逆转录酶作为MAPL阳性线粒体衍生囊泡形成的关键。基于聚乙二醇的细胞-细胞融合试验结果表明,MAPL 在过氧化物酶体中的富集是通过囊泡和逆转录酶依赖性现象。因此,建立了 MAPL 在过氧化物酶体中的新功能,以在生长条件下调节过氧化物酶体的伸长和形态,从而可能调节过氧化物酶体的裂变。
更新日期:2021-07-28
中文翻译:

线粒体锚定蛋白连接酶在哺乳动物细胞过氧化物酶体形态中的新功能作用
线粒体和过氧化物酶体是代谢相互关联且功能活跃的亚细胞器。这两个动态细胞器具有许多共同的生化功能,例如脂肪酸的β-氧化和过氧化物的解毒。哺乳动物细胞中这两种细胞器的生物发生和形态均由常见的转录因子(如 PGC1α)和由裂变蛋白(如 DRP1、Mff 和 hFis1)组成的常见裂变机制控制。此外,外膜线粒体锚定蛋白连接酶 (MAPL) 是第一个具有环指结构域的线粒体 SUMO E3 连接酶,它也调节线粒体形态,在其过表达时诱导线粒体断裂。这种片段化取决于 MAPL 的 RING 结构域和线粒体裂变 GTPase dynamin-related protein-1 (DRP1) 的存在。早期的研究表明,线粒体衍生的囊泡是独立于已知的线粒体裂变 GTPase 形成的,DRP1 富含 MAPL 并靶向过氧化物酶体。目前的研究表明,MAPL 以细胞类型特异性方式调节过氧化物酶体的形态。令人着迷的是,由于 DRP1 的沉默或通过添加多不饱和脂肪酸引起的过氧化物酶体延长,二十二碳六烯酸被哺乳动物细胞系中过表达的 MAPL 所阻断。此外,MAPL 与过氧化物酶体膜标记物 PMP70 的转染和共定位研究,在不同的细胞系中清楚地揭示了 MAPL 向过氧化物酶体转运的细胞类型特异性。以前的工作已将 Vps35(逆转录酶组分)作为将 MAPL 递送至过氧化物酶体的关键,将逆转录酶作为形成 MAPL 阳性线粒体衍生囊泡的关键。基于聚乙二醇的细胞-细胞融合试验结果表明,MAPL 在过氧化物酶体中的富集是通过囊泡和逆转录酶依赖性现象。因此,建立了 MAPL 在过氧化物酶体中的新功能,以在生长条件下调节过氧化物酶体的伸长和形态,从而可能调节过氧化物酶体的裂变。将逆转录酶作为MAPL阳性线粒体衍生囊泡形成的关键。基于聚乙二醇的细胞-细胞融合试验结果表明,MAPL 在过氧化物酶体中的富集是通过囊泡和逆转录酶依赖性现象。因此,建立了 MAPL 在过氧化物酶体中的新功能,以在生长条件下调节过氧化物酶体的伸长和形态,从而可能调节过氧化物酶体的裂变。将逆转录酶作为MAPL阳性线粒体衍生囊泡形成的关键。基于聚乙二醇的细胞-细胞融合试验结果表明,MAPL 在过氧化物酶体中的富集是通过囊泡和逆转录酶依赖性现象。因此,建立了 MAPL 在过氧化物酶体中的新功能,以在生长条件下调节过氧化物酶体的伸长和形态,从而可能调节过氧化物酶体的裂变。



























 京公网安备 11010802027423号
京公网安备 11010802027423号