Drug Discovery Today ( IF 7.4 ) Pub Date : 2021-07-29 , DOI: 10.1016/j.drudis.2021.07.021 Vivek P Chavda 1 , Lalitkumar K Vora 2 , Anjali K Pandya 3 , Vandana B Patravale 3
|
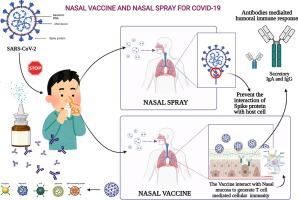
Unlike conventional Coronavirus 2019 (COVID-19) vaccines, intranasal vaccines display a superior advantage because the nasal mucosa is often the initial site of infection. Preclinical and clinical studies concerning intranasal immunization elicit high neutralizing antibody generation and mucosal IgA and T cell responses that avoid severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection in both; the upper and lower respiratory tract. A nasal formulation is non-invasive with high appeal to patients. Intranasal vaccines enable self-administration and can be designed to survive at ambient temperatures, thereby simplifying logistical aspects of transport and storage. In this review, we provide an overview of nasal vaccines with a focus on formulation development as well as ongoing preclinical and clinical studies for SARS-CoV-2 intranasal vaccine products.
中文翻译:

SARS-CoV-2 鼻内疫苗:从 COVID-19 管理的挑战到潜力
与传统的 2019 年冠状病毒 (COVID-19) 疫苗不同,鼻内疫苗显示出优越的优势,因为鼻粘膜通常是感染的初始部位。有关鼻内免疫的临床前和临床研究可引发高中和抗体生成以及粘膜 IgA 和 T 细胞反应,从而避免两者感染严重急性呼吸系统综合症冠状病毒 2 (SARS-CoV-2);上呼吸道和下呼吸道。鼻腔制剂是非侵入性的,对患者具有很高的吸引力。鼻内疫苗可以自我给药,并且可以设计成在环境温度下存活,从而简化运输和储存的后勤方面。在这篇评论中,



























 京公网安备 11010802027423号
京公网安备 11010802027423号