当前位置:
X-MOL 学术
›
FEBS Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Plant type I metacaspases are proteolytically active proteases despite their hydrophobic nature
FEBS Letters ( IF 3.5 ) Pub Date : 2021-07-28 , DOI: 10.1002/1873-3468.14165 Katarina Petra van Midden 1 , Tanja Peric 1 , Marina Klemenčič 1
FEBS Letters ( IF 3.5 ) Pub Date : 2021-07-28 , DOI: 10.1002/1873-3468.14165 Katarina Petra van Midden 1 , Tanja Peric 1 , Marina Klemenčič 1
Affiliation
|
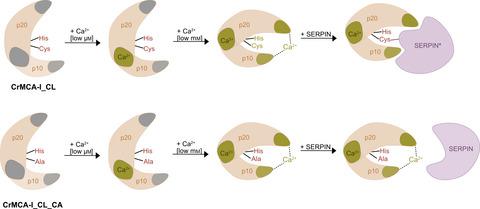
Plant metacaspases type I (MCA-Is), the closest structural homologs of caspases, are key proteases in stress-induced regulated cell death processes in plants. However, no plant MCA-Is have been characterized in vitro to date. Here, we show that only plant MCA-Is contain a highly hydrophobic loop within the C terminus of their p10 domain. When removed, soluble and proteolytically active plant MCA-Is can be designed and recombinantly produced. We show that the activity of MCA-I depends on calcium ions and that removal of the hydrophobic loop does not affect cleavage and covalent binding to its inhibitor SERPIN. This novel approach will finally allow the development of tools to detect and manipulate the activity of these cysteine proteases in vivo and in planta.
中文翻译:

尽管具有疏水性,植物 I 型间半胱天冬酶是具有蛋白水解活性的蛋白酶
植物转半胱天冬酶 I 型 (MCA-Is) 是半胱天冬酶最接近的结构同源物,是植物中胁迫诱导的受调节细胞死亡过程中的关键蛋白酶。然而,迄今为止,尚未在体外表征植物 MCA-Is 。在这里,我们表明只有植物 MCA-Is 在其 p10 域的 C 端包含一个高度疏水的环。去除后,可设计和重组生产可溶性和具有蛋白水解活性的植物 MCA-Is。我们表明 MCA-I 的活性取决于钙离子,并且去除疏水环不会影响裂解和与其抑制剂 SERPIN 的共价结合。这种新方法最终将允许开发工具来检测和操纵这些半胱氨酸蛋白酶在体内和植物中的活性。
更新日期:2021-09-13
中文翻译:

尽管具有疏水性,植物 I 型间半胱天冬酶是具有蛋白水解活性的蛋白酶
植物转半胱天冬酶 I 型 (MCA-Is) 是半胱天冬酶最接近的结构同源物,是植物中胁迫诱导的受调节细胞死亡过程中的关键蛋白酶。然而,迄今为止,尚未在体外表征植物 MCA-Is 。在这里,我们表明只有植物 MCA-Is 在其 p10 域的 C 端包含一个高度疏水的环。去除后,可设计和重组生产可溶性和具有蛋白水解活性的植物 MCA-Is。我们表明 MCA-I 的活性取决于钙离子,并且去除疏水环不会影响裂解和与其抑制剂 SERPIN 的共价结合。这种新方法最终将允许开发工具来检测和操纵这些半胱氨酸蛋白酶在体内和植物中的活性。



























 京公网安备 11010802027423号
京公网安备 11010802027423号