当前位置:
X-MOL 学术
›
Eur. J. Inorg. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Half-Sandwich (η6-Benzene)Ruthenium(II) Complex of Picolyl Functionalized N-Heterocyclic Carbene as an Efficient Catalyst for Thioether Directed C−H Alkenylation of Arenes
European Journal of Inorganic Chemistry ( IF 2.3 ) Pub Date : 2021-07-28 , DOI: 10.1002/ejic.202100501 Sangeeta Kumari 1 , Charu Sharma 2 , Avinash K Srivastava 2 , Naveen Satrawala 2 , Kamal N Sharma 2 , Raj Kumar Joshi 3
European Journal of Inorganic Chemistry ( IF 2.3 ) Pub Date : 2021-07-28 , DOI: 10.1002/ejic.202100501 Sangeeta Kumari 1 , Charu Sharma 2 , Avinash K Srivastava 2 , Naveen Satrawala 2 , Kamal N Sharma 2 , Raj Kumar Joshi 3
Affiliation
|
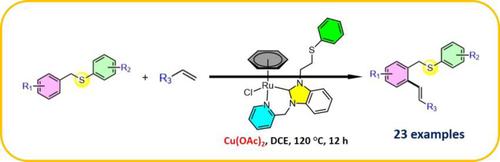
In this report, a half-sandwich (η6-benzene)Ru(II) complex of picolyl functionalized N-heterocyclic carbene was synthesized and efficiently used for the alkenylation arenes through thioether directed C−H bond activation. The thioether functionality of the substrate directed a selective ortho-vinylation through C−H bond activation. Moreover, reaction significantly works under mild reaction conditions than the previously reported ones which use the precious noble metal catalyst including the Pd(II), Rh(III), and Ir(III). Broad substrate scope using several benzyl thioethers and vinyl were found to be well tolerated for the present catalytic reaction to produces moderate to good yields of the desired products. Moreover, it is the first report where a Ru-based catalyst was used for the alkenylation of thioethers.
中文翻译:

吡啶甲基官能化的 N-杂环卡宾的半夹心(η6-苯)钌(II)配合物作为硫醚定向芳烃 C-H 烯基化的有效催化剂
在本报告中,合成了吡啶甲基官能化 N-杂环卡宾的半夹心(η 6 -苯)Ru(II)配合物,并通过硫醚定向的 CH 键活化有效地用于烯基化芳烃。底物的硫醚官能团定向选择性邻位- 通过 CH 键活化的乙烯基化。此外,与先前报道的使用包括 Pd(II)、Rh(III) 和 Ir(III) 在内的贵贵金属催化剂的反应相比,反应在温和的反应条件下显着进行。发现使用几种苄基硫醚和乙烯基的广泛底物范围对于本催化反应具有良好耐受性,以产生中等至良好产率的所需产物。此外,这是第一个使用 Ru 基催化剂进行硫醚烯基化的报道。
更新日期:2021-09-15
中文翻译:

吡啶甲基官能化的 N-杂环卡宾的半夹心(η6-苯)钌(II)配合物作为硫醚定向芳烃 C-H 烯基化的有效催化剂
在本报告中,合成了吡啶甲基官能化 N-杂环卡宾的半夹心(η 6 -苯)Ru(II)配合物,并通过硫醚定向的 CH 键活化有效地用于烯基化芳烃。底物的硫醚官能团定向选择性邻位- 通过 CH 键活化的乙烯基化。此外,与先前报道的使用包括 Pd(II)、Rh(III) 和 Ir(III) 在内的贵贵金属催化剂的反应相比,反应在温和的反应条件下显着进行。发现使用几种苄基硫醚和乙烯基的广泛底物范围对于本催化反应具有良好耐受性,以产生中等至良好产率的所需产物。此外,这是第一个使用 Ru 基催化剂进行硫醚烯基化的报道。



























 京公网安备 11010802027423号
京公网安备 11010802027423号