Journal of Environmental Chemical Engineering ( IF 7.7 ) Pub Date : 2021-07-28 , DOI: 10.1016/j.jece.2021.106126 Jean L.S. Fagundez 1 , Matias Schadeck Netto 1 , Guilherme L. Dotto 1 , Nina P.G. Salau 1
|
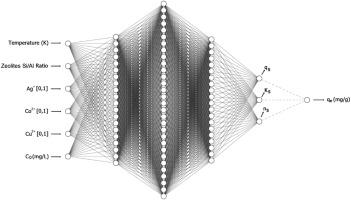
A new methodology for simultaneous parameter estimation of several adsorption curves through a hybrid ANN-isotherm model is proposed. For that, the ANN model was trained with 36 adsorption isotherm curves with three different adsorbates (Ag+, Co2+, and Cu2+), three different adsorbents (zeolites ZSM-5, HY, and 4 A), and four different temperatures. Two ANN-isotherm hybrid models were created. One was based on the Langmuir equation, and the other was based on Sips. A pseudo-outputs method was implemented for isotherm and thermodynamic parameters estimation. The hybrid models reached Pearson's linear correlation coefficient values between 0.98 and 1 for measured and predicted data. The Sips heterogeneity factor indicated that only zeolite 4 A performed the adsorption process with a certain heterogeneity degree. In contrast, zeolites ZSM-5 and HY had adsorptions fully described by the Langmuir isotherm. The assessment of adsorption thermodynamic parameters compared the isotherm-based values of equilibrium constant (K) and change in Gibbs' free energy (ΔG0) from the pseudo-outputs ANN structure to Van’t Hoff equations calculated K and ΔG0, showing good agreement with high values of R² and low values of RMSE. It was also possible to determine that zeolite 4 A showed the best adsorption efficiency due to the higher affinity with ions Ag+, Co2+, and Cu2+, followed by zeolite HY. Among the metallic ions, Ag+ and Cu2+ had similar adsorption behaviors onto all adsorbents, with improved adsorption as the temperature increased, while the Co2+ showed the lowest sensitivity to temperature variation.
中文翻译:

一种开发 ANN 等温线混合模型的新方法,用于确定离子 Ag+、Co2+ 和 Cu2+ 吸附到沸石 ZSM-5、HY 和 4A 上的热力学参数
提出了一种通过混合人工神经网络等温线模型同时估计多条吸附曲线参数的新方法。为此,ANN 模型使用 36 条吸附等温线进行训练,其中包含三种不同的吸附物(Ag +、Co 2+和 Cu 2+)、三种不同的吸附剂(沸石 ZSM-5、HY 和 4 A)以及四种不同的吸附剂。温度。创建了两个 ANN 等温线混合模型。一种基于朗缪尔方程,另一种基于 Sips。为等温线和热力学参数估计实施了伪输出方法。对于测量数据和预测数据,混合模型达到了介于 0.98 和 1 之间的 Pearson 线性相关系数值。Sips 异质性因子表明只有沸石 4 A进行了具有一定非均质性的吸附过程。相比之下,沸石 ZSM-5 和 HY 具有完全由朗缪尔等温线描述的吸附。吸附热力学参数的评估比较了基于等温线的平衡常数 ( K ) 值和 Gibbs 自由能 ( ΔG 0 ) 从伪输出 ANN 结构到 Van't Hoff 方程计算的K和ΔG 0 的变化,显示良好与R² 的高值和RMSE 的低值一致。还可以确定沸石 4 A 显示出最佳吸附效率,因为它与离子 Ag +、Co 2+、和Cu 2+,然后是沸石HY。在金属离子中,Ag +和 Cu 2+对所有吸附剂具有相似的吸附行为,随着温度的升高吸附性能提高,而 Co 2+对温度变化的敏感性最低。



























 京公网安备 11010802027423号
京公网安备 11010802027423号