当前位置:
X-MOL 学术
›
Chem. Res. Toxicol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Further Insights into the Oxidative Pathway of Thiocarbonyl-Type Antitubercular Prodrugs: Ethionamide, Thioacetazone, and Isoxyl
Chemical Research in Toxicology ( IF 4.1 ) Pub Date : 2021-07-28 , DOI: 10.1021/acs.chemrestox.1c00164 Tércio de Freitas Paulo 1, 2, 3 , Carine Duhayon 1, 2 , Luiz Gonzaga de França Lopes 3 , Eduardo Henrique Silva Sousa 3 , Remi Chauvin 1, 2 , Vania Bernardes-Génisson 1, 2
Chemical Research in Toxicology ( IF 4.1 ) Pub Date : 2021-07-28 , DOI: 10.1021/acs.chemrestox.1c00164 Tércio de Freitas Paulo 1, 2, 3 , Carine Duhayon 1, 2 , Luiz Gonzaga de França Lopes 3 , Eduardo Henrique Silva Sousa 3 , Remi Chauvin 1, 2 , Vania Bernardes-Génisson 1, 2
Affiliation
|
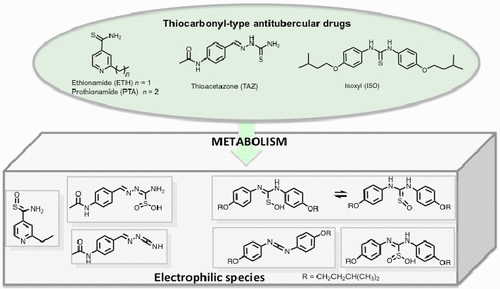
A chemical activation study of the thiocarbonyl-type antitubercular prodrugs, ethionamide (ETH), thioacetazone (TAZ), and isoxyl (ISO), was performed. Biomimetic oxidation of ethionamide using H2O2 (1 equiv) led to ETH–SO as the only stable S-oxide compound, which was found to occur in solution in the preferential form of a sulfine (ETH═S═O vs the sulfenic acid tautomer ETH–S–OH), as previously observed in the crystal state. It was also demonstrated that ETH–SO is capable of reacting with amines, as the putative sulfinic derivative (ETH–SO2H) was supposed to do. Unlike ETH, oxidation of TAZ did not allow observation of the mono-oxygenated species (TAZ–SO), leading directly to the more stable sulfinic acid derivative (TAZ–SO2H), which can then lose a SOxH group after further oxidation or when placed in a basic medium. It was also noticed that the unstable TAZ–SO intermediate can lead to the carbodiimide derivative as another electrophilic species. It is suggested that TAZ–SOH, TAZ–SO2H, and the carbodiimide compound can also react with NH2-containing nucleophilic species, and therefore be involved in toxic effects. Finally, ISO showed a very complex reactivity, here assigned to the coexistence of two mono-oxygenated structures, the sulfine and sulfenic acid tautomers. The mono- and dioxygenated derivatives of ISO are also highly unstable, leading to a panel of multiple metabolites, which are still reactive and likely contribute to the toxicity of this prodrug.
中文翻译:

进一步深入了解硫羰基型抗结核前药的氧化途径:乙硫异烟胺、硫乙酰酮和异氧基
对硫代羰基型抗结核前药乙硫异烟胺 (ETH)、硫代乙酰腙 (TAZ) 和异氧基 (ISO) 进行了化学活化研究。使用 H 2 O 2 (1 equiv)仿生氧化乙硫异烟胺导致 ETH-SO 作为唯一稳定的S-氧化物化合物,发现它以亚磺酸的优先形式出现在溶液中(ETH=S=O vs酸互变异构体 ETH-S-OH),如先前在晶体状态中观察到的。还证明 ETH-SO 能够与胺反应,正如假定的亚磺酸衍生物 (ETH-SO 2 H) 应该做的那样。与 ETH 不同,TAZ 的氧化不允许观察单氧化物质 (TAZ-SO),直接导致更稳定的亚磺酸衍生物 (TAZ-SO)2 H),在进一步氧化后或置于碱性介质中时,会失去 SO x H 基团。还注意到不稳定的 TAZ-SO 中间体会导致碳二亚胺衍生物作为另一种亲电物质。表明 TAZ-SOH、TAZ-SO 2 H 和碳二亚胺化合物也可以与含NH 2的亲核物质反应,因此参与毒性作用。最后,ISO 表现出非常复杂的反应性,这里归因于两种单氧化结构的共存,即亚磺酸和亚磺酸互变异构体。ISO 的单加氧和双加氧衍生物也非常不稳定,导致产生一组多种代谢物,这些代谢物仍然具有反应性并可能导致该前药的毒性。
更新日期:2021-08-16
中文翻译:

进一步深入了解硫羰基型抗结核前药的氧化途径:乙硫异烟胺、硫乙酰酮和异氧基
对硫代羰基型抗结核前药乙硫异烟胺 (ETH)、硫代乙酰腙 (TAZ) 和异氧基 (ISO) 进行了化学活化研究。使用 H 2 O 2 (1 equiv)仿生氧化乙硫异烟胺导致 ETH-SO 作为唯一稳定的S-氧化物化合物,发现它以亚磺酸的优先形式出现在溶液中(ETH=S=O vs酸互变异构体 ETH-S-OH),如先前在晶体状态中观察到的。还证明 ETH-SO 能够与胺反应,正如假定的亚磺酸衍生物 (ETH-SO 2 H) 应该做的那样。与 ETH 不同,TAZ 的氧化不允许观察单氧化物质 (TAZ-SO),直接导致更稳定的亚磺酸衍生物 (TAZ-SO)2 H),在进一步氧化后或置于碱性介质中时,会失去 SO x H 基团。还注意到不稳定的 TAZ-SO 中间体会导致碳二亚胺衍生物作为另一种亲电物质。表明 TAZ-SOH、TAZ-SO 2 H 和碳二亚胺化合物也可以与含NH 2的亲核物质反应,因此参与毒性作用。最后,ISO 表现出非常复杂的反应性,这里归因于两种单氧化结构的共存,即亚磺酸和亚磺酸互变异构体。ISO 的单加氧和双加氧衍生物也非常不稳定,导致产生一组多种代谢物,这些代谢物仍然具有反应性并可能导致该前药的毒性。



























 京公网安备 11010802027423号
京公网安备 11010802027423号