当前位置:
X-MOL 学术
›
Bioconjugate Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
An Overview of Recent Advances in Biomedical Applications of Click Chemistry
Bioconjugate Chemistry ( IF 4.7 ) Pub Date : 2021-07-28 , DOI: 10.1021/acs.bioconjchem.1c00247 Jasleen Kaur 1 , Mokshika Saxena 1 , Narayan Rishi 1
Bioconjugate Chemistry ( IF 4.7 ) Pub Date : 2021-07-28 , DOI: 10.1021/acs.bioconjchem.1c00247 Jasleen Kaur 1 , Mokshika Saxena 1 , Narayan Rishi 1
Affiliation
|
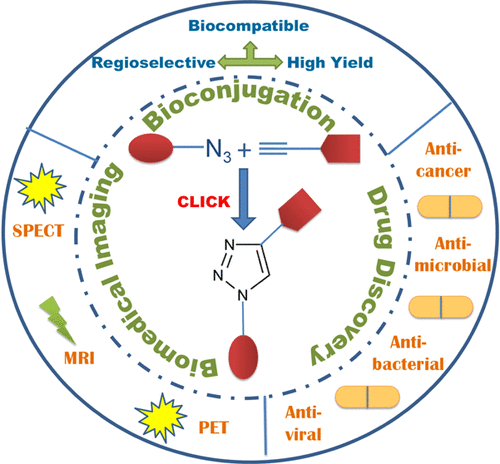
Cu(I)-catalyzed azide–alkyne cycloaddition (CuAAC) is a modular and bio-orthogonal approach that is being adopted for the efficient synthesis of organic and bioorganic compounds. It leads to the selective formation of 1,4-disubstituted 1,2,3-triazole units connecting readily accessible building blocks via a stable and biocompatible linkage. The vast array of the bioconjugation applications of click chemistry has been attributed to its fast reaction kinetics, quantitative yields, minimal byproducts, and high chemospecificity and regioselectivity. These combined advantages make click reactions quite suitable for the lead identification and the development of pharmaceutical agents in the fields of medicinal chemistry and drug discovery. In this review, we have outlined the key aspects, the mechanistic details and merits and demerits of the click reaction. In addition, we have also discussed the recent pharmaceutical applications of click chemistry, ranging from the development of anticancer, antibacterial, and antiviral agents to that of biomedical imaging agents and clinical therapeutics.
中文翻译:

点击化学生物医学应用的最新进展概述
Cu(I)催化的叠氮化物 - 炔烃环加成(CuAAC)是一种模块化和生物正交方法,被用于有效合成有机和生物有机化合物。它导致选择性形成 1,4-二取代的 1,2,3-三唑单元,通过稳定和生物相容的连接连接容易获得的构件。点击化学的大量生物偶联应用归因于其快速反应动力学、定量产率、最少的副产物以及高化学特异性和区域选择性。这些综合优势使点击反应非常适用于药物化学和药物发现领域的先导鉴定和药物制剂的开发。在本次审查中,我们概述了关键方面,点击反应的机制细节和优缺点。此外,我们还讨论了点击化学最近的药物应用,从抗癌、抗菌和抗病毒药物的开发到生物医学显像剂和临床治疗的开发。
更新日期:2021-08-19
中文翻译:

点击化学生物医学应用的最新进展概述
Cu(I)催化的叠氮化物 - 炔烃环加成(CuAAC)是一种模块化和生物正交方法,被用于有效合成有机和生物有机化合物。它导致选择性形成 1,4-二取代的 1,2,3-三唑单元,通过稳定和生物相容的连接连接容易获得的构件。点击化学的大量生物偶联应用归因于其快速反应动力学、定量产率、最少的副产物以及高化学特异性和区域选择性。这些综合优势使点击反应非常适用于药物化学和药物发现领域的先导鉴定和药物制剂的开发。在本次审查中,我们概述了关键方面,点击反应的机制细节和优缺点。此外,我们还讨论了点击化学最近的药物应用,从抗癌、抗菌和抗病毒药物的开发到生物医学显像剂和临床治疗的开发。



























 京公网安备 11010802027423号
京公网安备 11010802027423号