当前位置:
X-MOL 学术
›
Mol. Pharmacol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Acylation of the Incretin Peptide Exendin-4 Directly Impacts Glucagon-Like Peptide-1 Receptor Signaling and Trafficking
Molecular Pharmacology ( IF 3.6 ) Pub Date : 2021-10-01 , DOI: 10.1124/molpharm.121.000270 Maria Lucey 1 , Tanyel Ashik 1 , Amaara Marzook 1 , Yifan Wang 1 , Joëlle Goulding 1 , Atsuro Oishi 1 , Johannes Broichhagen 1 , David J Hodson 1 , James Minnion 1 , Yuval Elani 1 , Ralf Jockers 1 , Stephen J Briddon 1 , Stephen R Bloom 1 , Alejandra Tomas 2 , Ben Jones 2
Molecular Pharmacology ( IF 3.6 ) Pub Date : 2021-10-01 , DOI: 10.1124/molpharm.121.000270 Maria Lucey 1 , Tanyel Ashik 1 , Amaara Marzook 1 , Yifan Wang 1 , Joëlle Goulding 1 , Atsuro Oishi 1 , Johannes Broichhagen 1 , David J Hodson 1 , James Minnion 1 , Yuval Elani 1 , Ralf Jockers 1 , Stephen J Briddon 1 , Stephen R Bloom 1 , Alejandra Tomas 2 , Ben Jones 2
Affiliation
|
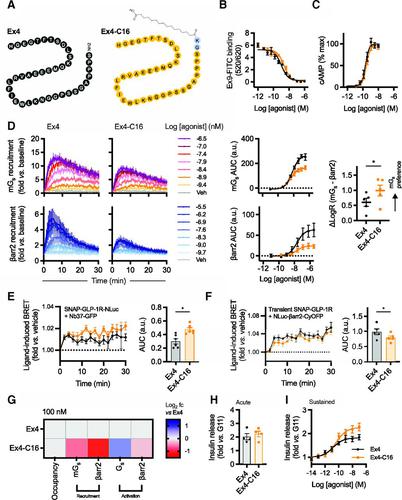
The glucagon-like peptide-1 receptor (GLP-1R) is a class B G protein–coupled receptor and mainstay therapeutic target for the treatment of type 2 diabetes and obesity. Recent reports have highlighted how biased agonism at the GLP-1R affects sustained glucose-stimulated insulin secretion through avoidance of desensitization and downregulation. A number of GLP-1R agonists (GLP-1RAs) feature a fatty acid moiety to prolong their pharmacokinetics via increased albumin binding, but the potential for these chemical changes to influence GLP-1R function has rarely been investigated beyond potency assessments for cAMP. Here, we directly compare the prototypical GLP-1RA exendin-4 with its C-terminally acylated analog, exendin-4-C16. We examine relative propensities of each ligand to recruit and activate G proteins and β-arrestins, endocytic and postendocytic trafficking profiles, and interactions with model and cellular membranes in HEK293 and HEK293T cells. Both ligands had similar cAMP potency, but exendin-4-C16 showed ∼2.5-fold bias toward G protein recruitment and a ∼60% reduction in β-arrestin-2 recruitment efficacy compared with exendin-4, as well as reduced GLP-1R endocytosis and preferential targeting toward recycling pathways. These effects were associated with reduced movement of the GLP-1R extracellular domain measured using a conformational biosensor approach and a ∼70% increase in insulin secretion in INS-1 832/3 cells. Interactions with plasma membrane lipids were enhanced by the acyl chain. Exendin-4-C16 showed extensive albumin binding and was highly effective for lowering of blood glucose in mice over at least 72 hours. Our study highlights the importance of a broad approach to the evaluation of GLP-1RA pharmacology.
中文翻译:

肠促胰岛素肽 Exendin-4 的酰化直接影响胰高血糖素样肽-1 受体信号传导和运输
胰高血糖素样肽-1 受体 (GLP-1R) 是一种 BG 蛋白偶联受体,也是治疗 2 型糖尿病和肥胖症的主要治疗靶点。最近的报告强调了 GLP-1R 的偏向激动如何通过避免脱敏和下调来影响持续的葡萄糖刺激的胰岛素分泌。许多 GLP-1R 激动剂 (GLP-1RA) 具有脂肪酸部分,可通过增加白蛋白结合来延长其药代动力学,但除了 cAMP 的效力评估之外,很少研究这些化学变化影响 GLP-1R 功能的潜力。在这里,我们直接比较原型 GLP-1RA exendin-4 与其 C 末端酰化类似物 exendin-4-C16。我们检查了每个配体招募和激活 G 蛋白和β-抑制蛋白的相对倾向、内吞和内吞后运输概况,以及 HEK293 和 HEK293T 细胞中与模型和细胞膜的相互作用。两种配体具有相似的 cAMP 效力,但与 exendin-4 相比,exendin-4-C16 对 G 蛋白招募有约 2.5 倍的偏向,并且β -arrestin-2 招募功效降低约 60%,并且 GLP-1R 减少内吞作用和优先靶向回收途径。这些效应与使用构象生物传感器方法测量的 GLP-1R 胞外结构域运动减少以及 INS-1 832/3 细胞中胰岛素分泌增加约 70% 相关。酰基链增强了与质膜脂质的相互作用。Exendin-4-C16 显示出广泛的白蛋白结合,并且在至少 72 小时内对降低小鼠血糖非常有效。我们的研究强调了采用广泛方法评估 GLP-1RA 药理学的重要性。
更新日期:2021-10-06
中文翻译:

肠促胰岛素肽 Exendin-4 的酰化直接影响胰高血糖素样肽-1 受体信号传导和运输
胰高血糖素样肽-1 受体 (GLP-1R) 是一种 BG 蛋白偶联受体,也是治疗 2 型糖尿病和肥胖症的主要治疗靶点。最近的报告强调了 GLP-1R 的偏向激动如何通过避免脱敏和下调来影响持续的葡萄糖刺激的胰岛素分泌。许多 GLP-1R 激动剂 (GLP-1RA) 具有脂肪酸部分,可通过增加白蛋白结合来延长其药代动力学,但除了 cAMP 的效力评估之外,很少研究这些化学变化影响 GLP-1R 功能的潜力。在这里,我们直接比较原型 GLP-1RA exendin-4 与其 C 末端酰化类似物 exendin-4-C16。我们检查了每个配体招募和激活 G 蛋白和β-抑制蛋白的相对倾向、内吞和内吞后运输概况,以及 HEK293 和 HEK293T 细胞中与模型和细胞膜的相互作用。两种配体具有相似的 cAMP 效力,但与 exendin-4 相比,exendin-4-C16 对 G 蛋白招募有约 2.5 倍的偏向,并且β -arrestin-2 招募功效降低约 60%,并且 GLP-1R 减少内吞作用和优先靶向回收途径。这些效应与使用构象生物传感器方法测量的 GLP-1R 胞外结构域运动减少以及 INS-1 832/3 细胞中胰岛素分泌增加约 70% 相关。酰基链增强了与质膜脂质的相互作用。Exendin-4-C16 显示出广泛的白蛋白结合,并且在至少 72 小时内对降低小鼠血糖非常有效。我们的研究强调了采用广泛方法评估 GLP-1RA 药理学的重要性。



























 京公网安备 11010802027423号
京公网安备 11010802027423号