当前位置:
X-MOL 学术
›
Org. Process Res. Dev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Synthesis of Enantiopure Unnatural Amino Acids by Metallaphotoredox Catalysis
Organic Process Research & Development ( IF 3.4 ) Pub Date : 2021-07-26 , DOI: 10.1021/acs.oprd.1c00208 Tomer M Faraggi 1 , Caroline Rouget-Virbel 1 , Juan A Rincón 2 , Mario Barberis 2 , Carlos Mateos 2 , Susana García-Cerrada 2 , Javier Agejas 2 , Oscar de Frutos 2 , David W C MacMillan 1
Organic Process Research & Development ( IF 3.4 ) Pub Date : 2021-07-26 , DOI: 10.1021/acs.oprd.1c00208 Tomer M Faraggi 1 , Caroline Rouget-Virbel 1 , Juan A Rincón 2 , Mario Barberis 2 , Carlos Mateos 2 , Susana García-Cerrada 2 , Javier Agejas 2 , Oscar de Frutos 2 , David W C MacMillan 1
Affiliation
|
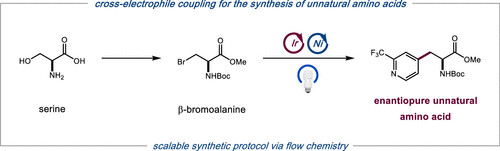
We describe herein a two-step process for the conversion of serine to a wide array of optically pure unnatural amino acids. This method utilizes a photocatalytic cross-electrophile coupling between a bromoalkyl intermediate and a diverse set of aryl halides to produce artificial analogues of phenylalanine, tryptophan, and histidine. The reaction is tolerant of a broad range of functionalities and can be leveraged toward the scalable synthesis of valuable pharmaceutical scaffolds via flow technology.
中文翻译:

金属光氧化还原催化合成对映体纯非天然氨基酸
我们在本文中描述了将丝氨酸转化为多种光学纯非天然氨基酸的两步过程。该方法利用溴代烷基中间体和多种芳基卤化物之间的光催化交叉亲电偶联来生产苯丙氨酸、色氨酸和组氨酸的人工类似物。该反应具有广泛的功能,可用于通过流动技术可扩展地合成有价值的药物支架。
更新日期:2021-08-20
中文翻译:

金属光氧化还原催化合成对映体纯非天然氨基酸
我们在本文中描述了将丝氨酸转化为多种光学纯非天然氨基酸的两步过程。该方法利用溴代烷基中间体和多种芳基卤化物之间的光催化交叉亲电偶联来生产苯丙氨酸、色氨酸和组氨酸的人工类似物。该反应具有广泛的功能,可用于通过流动技术可扩展地合成有价值的药物支架。



























 京公网安备 11010802027423号
京公网安备 11010802027423号