当前位置:
X-MOL 学术
›
Mol. Pharmaceutics
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Prediction of the Synergistic Glass Transition Temperature of Coamorphous Molecular Glasses Using Activity Coefficient Models
Molecular Pharmaceutics ( IF 4.9 ) Pub Date : 2021-07-27 , DOI: 10.1021/acs.molpharmaceut.1c00353 Xiao Zhao 1 , Sixue Cheng 1 , Yung P Koh 1 , Brandon D Kelly 1 , Gregory B McKenna 1, 2 , Sindee L Simon 1, 2
Molecular Pharmaceutics ( IF 4.9 ) Pub Date : 2021-07-27 , DOI: 10.1021/acs.molpharmaceut.1c00353 Xiao Zhao 1 , Sixue Cheng 1 , Yung P Koh 1 , Brandon D Kelly 1 , Gregory B McKenna 1, 2 , Sindee L Simon 1, 2
Affiliation
|
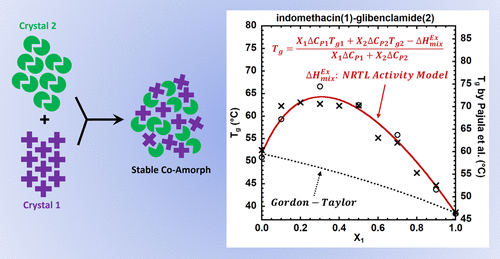
The glass transition temperature (Tg) of a binary miscible mixture of molecular glasses, termed a coamorphous glass, is often synergistically increased over that expected for an athermal mixture due to the strong interactions between the two components. This synergistic interaction is particularly important for the formulation of coamorphous pharmaceuticals since the molecular interactions and resulting Tg strongly impact stability against crystallization, dissolution kinetics, and bioavailability. Current models that describe the composition dependence of Tg for binary systems, including the Gordon–Taylor, Fox, Kwei, and Braun–Kovacs equations, fail to describe the behavior of coamorphous pharmaceuticals using parameters consistent with experimental ΔCP and Δα. Here, we develop a robust thermodynamic approach extending the Couchman and Karasz method through the use of activity coefficient models, including the two-parameter Margules, non-random-two-liquid (NRTL), and three-suffix Redlich–Kister models. We find that the models, using experimental values of ΔCP and fitting parameters related to the binary interactions, successfully describe observed synergistic elevations and inflections in the Tg versus composition response of coamorphous pharmaceuticals. Moreover, the predictions from the NRTL model are improved when the association-NRTL version of that model is used. Results are reported and discussed for four different coamorphous systems: indomethacin–glibenclamide, indomethacin–arginine, acetaminophen–indomethacin, and fenretinide–cholic acid.
中文翻译:

使用活度系数模型预测共晶分子玻璃的协同玻璃化转变温度
由于两种组分之间的强相互作用,分子玻璃的二元混溶混合物(称为无定形玻璃)的玻璃化转变温度 ( T g ) 通常比无热混合物的预期值协同增加。这种协同相互作用对于共晶药物的配方尤为重要,因为分子相互作用和产生的T g强烈影响结晶稳定性、溶解动力学和生物利用度。当前描述T g成分依赖性的模型对于二元系统,包括 Gordon-Taylor、Fox、 Kwei和 Braun-Kovacs 方程,未能使用与实验 ΔCP和 Δα一致的参数来描述同晶药物的行为。在这里,我们开发了一种稳健的热力学方法,通过使用活动系数模型来扩展 Couchman 和 Karasz 方法,包括两参数 Margules、非随机二液体 (NRTL) 和三后缀 Redlich-Kister 模型。我们发现,使用 ΔCP 的实验值和与二元相互作用相关的拟合参数的模型成功地描述了在T g中观察到的协同升高和拐点与无定形药物的成分反应相比。此外,当使用该模型的关联-NRTL 版本时,来自 NRTL 模型的预测得到改进。报告并讨论了四种不同的无定形系统的结果:消炎痛-格列本脲、消炎痛-精氨酸、对乙酰氨基酚-消炎痛和芬维A胺-胆酸。
更新日期:2021-09-06
中文翻译:

使用活度系数模型预测共晶分子玻璃的协同玻璃化转变温度
由于两种组分之间的强相互作用,分子玻璃的二元混溶混合物(称为无定形玻璃)的玻璃化转变温度 ( T g ) 通常比无热混合物的预期值协同增加。这种协同相互作用对于共晶药物的配方尤为重要,因为分子相互作用和产生的T g强烈影响结晶稳定性、溶解动力学和生物利用度。当前描述T g成分依赖性的模型对于二元系统,包括 Gordon-Taylor、Fox、 Kwei和 Braun-Kovacs 方程,未能使用与实验 ΔCP和 Δα一致的参数来描述同晶药物的行为。在这里,我们开发了一种稳健的热力学方法,通过使用活动系数模型来扩展 Couchman 和 Karasz 方法,包括两参数 Margules、非随机二液体 (NRTL) 和三后缀 Redlich-Kister 模型。我们发现,使用 ΔCP 的实验值和与二元相互作用相关的拟合参数的模型成功地描述了在T g中观察到的协同升高和拐点与无定形药物的成分反应相比。此外,当使用该模型的关联-NRTL 版本时,来自 NRTL 模型的预测得到改进。报告并讨论了四种不同的无定形系统的结果:消炎痛-格列本脲、消炎痛-精氨酸、对乙酰氨基酚-消炎痛和芬维A胺-胆酸。



























 京公网安备 11010802027423号
京公网安备 11010802027423号