Nano Research ( IF 9.9 ) Pub Date : 2021-07-27 , DOI: 10.1007/s12274-021-3626-2 Xiaoqing Yi 1 , Weijia Zeng 1 , Cui Wang 1 , Ying Chen 1 , Liangyuan Zheng 1 , Xinlin Zhu 1 , Ying Kuang 1 , Qitong Huang 1 , Yuqiu Ke 2 , Xiaoyan He 3
|
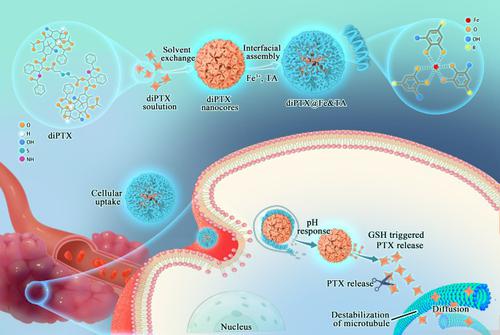
Currently, chemotherapy is the main clinical therapy of tumors. Depressingly, most chemotherapeutic drugs such as doxorubicin and paclitaxel (PTX) have poor water solubility, leading to low bioavailability and serious side effects. Till now, although a variety of nanoparticulate drug delivery systems have been designed to ameliorate the above disadvantage of chemotherapy drugs, their application is still severely limited due to the complex preparation, poor stability, low drug loading, and premature drug release. Herein, a metal phenolic network-based drug delivery system with superior stability, satisfactory drug loading capacity, good biocompatibility, reduced undesired premature release, and excellent anti-tumor ability has been established for achieving step-by-step multiple stimuli-responsive drug delivery. Firstly, the redox-responsive dimeric paclitaxel (diPTX) prodrug was synthesized. Then diPTX@Fe&tannic acid (diPTX@Fe&TA) complex nanoparticles with satisfactory PTX loading capacity were obtained by deposition of Fe&TA network complex on the nanocore of diPTX rapidly with a simple method. The diPTX@Fe&TA nanoparticles have a hydrodynamic diameter of 152.6 ± 1.2 nm, long-term colloidal stability, and high PTX loading content of 24.7%. Besides, diPTX@Fe&TA could expose to the acidic lysosomal environment and the reduction cytoplasmic environment continuously, resulting in the sequential release of diPTX and PTX when it was phagocytosed by tumor cells. Meanwhile, PTX showed almost no release under physiological condition (pH 7.4), which effectively inhibited the undesirable premature release of PTX. More importantly, diPTX@Fe&TA could suppress the growth of tumor effectively in vivo, along with negligible toxicity for organs. This work developed a simple and novel approach for the construction of a stepwise multiple stimuli-responsive drug delivery system with superior stability and satisfactory drug loading capacity to inhibit tumor growth effectively.
中文翻译:

用于化疗的分步多刺激响应金属-酚类网络前药纳米粒子
目前,化疗是肿瘤的主要临床治疗方法。令人沮丧的是,大多数化疗药物如阿霉素和紫杉醇 (PTX) 的水溶性较差,导致生物利用度低和严重的副作用。迄今为止,虽然已经设计了多种纳米颗粒给药系统来改善化疗药物的上述缺点,但由于制剂复杂、稳定性差、载药量低、药物释放过早等问题,其应用仍然受到严重限制。在此,建立了一种稳定性优异、载药量令人满意、生物相容性好、不良过早释放减少、抗肿瘤能力强的基于金属酚网络的递药系统,以实现分步多刺激响应递药。 . 首先,合成了氧化还原反应性二聚紫杉醇 (diPTX) 前药。然后通过简单的方法将Fe&TA网络复合物快速沉积在diPTX的纳米核上,获得了具有令人满意的PTX负载能力的diPTX@Fe&tannic acid(diPTX@Fe&TA)复合纳米颗粒。diPTX@Fe&TA 纳米粒子的流体力学直径为 152.6 ± 1.2 nm,胶体长期稳定,PTX 负载量高达 24.7%。此外,diPTX@Fe&TA可以持续暴露于酸性溶酶体环境和还原性细胞质环境,导致diPTX和PTX在被肿瘤细胞吞噬时依次释放。同时,PTX在生理条件(pH 7.4)下几乎不释放,有效抑制了PTX的过早释放。更重要的是,diPTX@Fe&在体内,对器官的毒性可以忽略不计。这项工作开发了一种简单而新颖的方法,用于构建具有优异稳定性和令人满意的载药量的逐步多刺激响应药物递送系统,以有效抑制肿瘤生长。



























 京公网安备 11010802027423号
京公网安备 11010802027423号