当前位置:
X-MOL 学术
›
Chem. Bio. Drug Des.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Radiosynthesis and in silico bioevaluation of 131I-Sulfasalazine as a highly selective radiotracer for imaging of ulcerative colitis
Chemical Biology & Drug Design ( IF 3 ) Pub Date : 2021-07-27 , DOI: 10.1111/cbdd.13929 M H Sanad 1 , Syed Faheem Askari Rizvi 2 , Ayman B Farag 3
Chemical Biology & Drug Design ( IF 3 ) Pub Date : 2021-07-27 , DOI: 10.1111/cbdd.13929 M H Sanad 1 , Syed Faheem Askari Rizvi 2 , Ayman B Farag 3
Affiliation
|
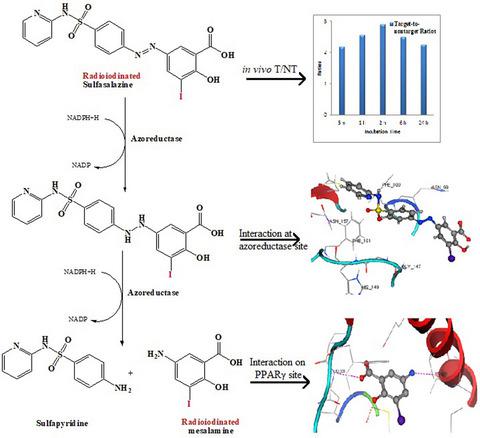
This study demonstrated the tracking of ulcerative colitis, which is considered a stressful immune disease. Although there are many ways to test for this disease including dependence on gases, dyes, and painful anal endoscopy, these treatment modalities have many disadvantages. Hence, it is the utmost need of time to discover new methods to detect this chronic immune disease and to avoid the defects of traditional methodologies. Sulfasalazine (SSD) was labeled with iodine-131 (half-life: 8 days, Energy: 971 keV) under optimum reaction conditions including the amount of reducing agent, pH factor, chloramine-T (Ch-T) amount, and incubation period. Characterization was performed using 1H/ 13C-NMR, ESI-MS, and HPLC (UV/ Radio) techniques. The biodistribution study was performed in normal and ulcerative mice models, and in silico molecular docking study was performed to evaluate the possible mechanism of action to target peroxisome proliferator-activated receptor gamma (PPARγ). The high radiolabeling yield of [131I]-sulfasalazine ([131I]-SSD) was achieved ≥90% through the direct labeling method with radioactive iodine-131 in the presence of chloramine-T (100 μg). The radiotracer [131I]-SSD was observed to be stable in normal saline and freshly eluted serum up to 12 hr at ambient temperature (37℃ ± 2℃). The radiotracer [131I]-SSD showed the highest uptake in the targeted organ (i.e., ulcerative colon) which was observed to be ≥75% injected dose per gram (% ID/g) organ for 24 hr postinjection (p.i). Furthermore, in silico data collected from molecular modeling analysis of SSD and [131I]-SSD with antimicrobial protein (PDB code: 3KEG) and peroxisome proliferator-activated receptor gamma (PPARγ) (PDB code: 4XTA) showed azoreductase activity and high binding potential for PPAR-γ site, respectively. The results of biological studies obtained in this study enlighten the usefulness of radiotracer [131I]-SSD as a potential imaging agent for ulcerative colitis.
中文翻译:

131I-柳氮磺胺吡啶作为溃疡性结肠炎成像的高选择性放射性示踪剂的放射合成和计算机生物评价
这项研究证明了溃疡性结肠炎的追踪,这被认为是一种压力性免疫疾病。尽管有很多方法可以检测这种疾病,包括依赖气体、染料和痛苦的肛门内窥镜检查,但这些治疗方式有很多缺点。因此,发现检测这种慢性免疫疾病的新方法并避免传统方法的缺陷是最需要时间的。在还原剂用量、pH因子、氯胺-T(Ch-T)用量、孵育期等最佳反应条件下,用碘131标记柳氮磺胺吡啶(SSD)(半衰期:8天,能量:971 keV) . 使用1 H/ 13进行表征C-NMR、ESI-MS 和 HPLC(紫外/无线电)技术。在正常和溃疡性小鼠模型中进行了生物分布研究,并进行了计算机分子对接研究,以评估靶向过氧化物酶体增殖物激活受体 γ (PPARγ) 的可能作用机制。[ 131 I]-柳氮磺胺吡啶([ 131 I]-SSD)在氯胺-T(100 μg)存在下通过放射性碘131直接标记的方法实现了≥90%的高放射性标记产率。观察到放射性示踪剂 [ 131 I]-SSD 在环境温度 (37℃ ± 2℃) 下在生理盐水和新鲜洗脱的血清中保持稳定长达 12 小时。放射性示踪剂 [ 131I]-SSD 显示在目标器官(即溃疡性结肠)中的摄取最高,在注射后 24 小时 (pi) 观察到每克器官的注射剂量≥75% (% ID/g)。此外,从 SSD 和 [ 131 I]-SSD 与抗菌蛋白(PDB 代码:3KEG)和过氧化物酶体增殖物激活受体 γ(PPARγ)(PDB 代码:4XTA)的分子建模分析收集的计算机数据显示偶氮还原酶活性和高结合PPAR-γ位点的潜力,分别。本研究中获得的生物学研究结果启发了放射性示踪剂 [ 131 I]-SSD 作为溃疡性结肠炎的潜在显像剂的有用性。
更新日期:2021-07-27
中文翻译:

131I-柳氮磺胺吡啶作为溃疡性结肠炎成像的高选择性放射性示踪剂的放射合成和计算机生物评价
这项研究证明了溃疡性结肠炎的追踪,这被认为是一种压力性免疫疾病。尽管有很多方法可以检测这种疾病,包括依赖气体、染料和痛苦的肛门内窥镜检查,但这些治疗方式有很多缺点。因此,发现检测这种慢性免疫疾病的新方法并避免传统方法的缺陷是最需要时间的。在还原剂用量、pH因子、氯胺-T(Ch-T)用量、孵育期等最佳反应条件下,用碘131标记柳氮磺胺吡啶(SSD)(半衰期:8天,能量:971 keV) . 使用1 H/ 13进行表征C-NMR、ESI-MS 和 HPLC(紫外/无线电)技术。在正常和溃疡性小鼠模型中进行了生物分布研究,并进行了计算机分子对接研究,以评估靶向过氧化物酶体增殖物激活受体 γ (PPARγ) 的可能作用机制。[ 131 I]-柳氮磺胺吡啶([ 131 I]-SSD)在氯胺-T(100 μg)存在下通过放射性碘131直接标记的方法实现了≥90%的高放射性标记产率。观察到放射性示踪剂 [ 131 I]-SSD 在环境温度 (37℃ ± 2℃) 下在生理盐水和新鲜洗脱的血清中保持稳定长达 12 小时。放射性示踪剂 [ 131I]-SSD 显示在目标器官(即溃疡性结肠)中的摄取最高,在注射后 24 小时 (pi) 观察到每克器官的注射剂量≥75% (% ID/g)。此外,从 SSD 和 [ 131 I]-SSD 与抗菌蛋白(PDB 代码:3KEG)和过氧化物酶体增殖物激活受体 γ(PPARγ)(PDB 代码:4XTA)的分子建模分析收集的计算机数据显示偶氮还原酶活性和高结合PPAR-γ位点的潜力,分别。本研究中获得的生物学研究结果启发了放射性示踪剂 [ 131 I]-SSD 作为溃疡性结肠炎的潜在显像剂的有用性。


























 京公网安备 11010802027423号
京公网安备 11010802027423号