当前位置:
X-MOL 学术
›
Chem. Rev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Catalytic Enantioselective Alkylation of Prochiral Enolates
Chemical Reviews ( IF 62.1 ) Pub Date : 2021-07-26 , DOI: 10.1021/acs.chemrev.0c00564 Timothy B Wright 1 , P Andrew Evans 1, 2
Chemical Reviews ( IF 62.1 ) Pub Date : 2021-07-26 , DOI: 10.1021/acs.chemrev.0c00564 Timothy B Wright 1 , P Andrew Evans 1, 2
Affiliation
|
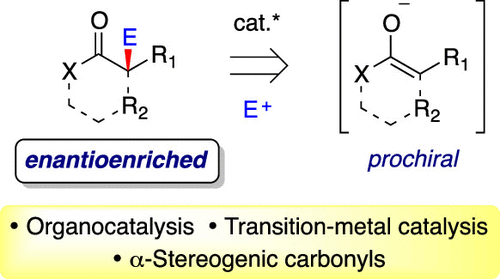
The asymmetric alkylation of enolates is a particularly versatile method for the construction of α-stereogenic carbonyl motifs, which are ubiquitous in synthetic chemistry. Over the past several decades, the focus has shifted to the development of new catalytic methods that depart from classical stoichiometric stereoinduction strategies (e.g., chiral auxiliaries, chiral alkali metal amide bases, chiral electrophiles, etc.). In this way, the enantioselective alkylation of prochiral enolates greatly improves the step- and redox-economy of this process, in addition to enhancing the scope and selectivity of these reactions. In this review, we summarize the origin and advancement of catalytic enantioselective enolate alkylation methods, with a directed emphasis on the union of prochiral nucleophiles with carbon-centered electrophiles for the construction of α-stereogenic carbonyl derivatives. Hence, the transformative developments for each distinct class of nucleophile (e.g., ketone enolates, ester enolates, amide enolates, etc.) are presented in a modular format to highlight the state-of-the-art methods and current limitations in each area.
中文翻译:

前手性烯醇化物的催化对映选择性烷基化
烯醇化物的不对称烷基化是构建α-立体羰基基序的一种特别通用的方法,该基序在合成化学中普遍存在。在过去的几十年中,重点已经转移到开发新的催化方法,这些方法与经典的化学计量立体诱导策略不同(例如,手性助剂、手性碱金属酰胺碱、手性亲电试剂等)。通过这种方式,前手性烯醇化物的对映选择性烷基化除了提高这些反应的范围和选择性外,还极大地改善了该过程的分步经济和氧化还原经济。在这篇综述中,我们总结了催化对映选择性烯醇烷基化方法的起源和进展,重点强调了具有碳中心亲电子试剂的前手性亲核试剂用于构建α-立体羰基衍生物。因此,每个不同类别的亲核试剂(例如,酮烯醇化物、酯烯醇化物、酰胺烯醇化物等)的变革性发展都以模块化格式呈现,以突出每个领域的最新方法和当前限制。
更新日期:2021-08-11
中文翻译:

前手性烯醇化物的催化对映选择性烷基化
烯醇化物的不对称烷基化是构建α-立体羰基基序的一种特别通用的方法,该基序在合成化学中普遍存在。在过去的几十年中,重点已经转移到开发新的催化方法,这些方法与经典的化学计量立体诱导策略不同(例如,手性助剂、手性碱金属酰胺碱、手性亲电试剂等)。通过这种方式,前手性烯醇化物的对映选择性烷基化除了提高这些反应的范围和选择性外,还极大地改善了该过程的分步经济和氧化还原经济。在这篇综述中,我们总结了催化对映选择性烯醇烷基化方法的起源和进展,重点强调了具有碳中心亲电子试剂的前手性亲核试剂用于构建α-立体羰基衍生物。因此,每个不同类别的亲核试剂(例如,酮烯醇化物、酯烯醇化物、酰胺烯醇化物等)的变革性发展都以模块化格式呈现,以突出每个领域的最新方法和当前限制。


























 京公网安备 11010802027423号
京公网安备 11010802027423号