当前位置:
X-MOL 学术
›
Cryst. Growth Des.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Two-Component Molecular Crystals: What Is the Difference between Drug–Drug, Drug–GRAS, and CF–CF Databases? Evaluation of Melting Points and Ideal Solubility of Unknown Co-crystals
Crystal Growth & Design ( IF 3.8 ) Pub Date : 2021-07-26 , DOI: 10.1021/acs.cgd.1c00477 German L. Perlovich 1
Crystal Growth & Design ( IF 3.8 ) Pub Date : 2021-07-26 , DOI: 10.1021/acs.cgd.1c00477 German L. Perlovich 1
Affiliation
|
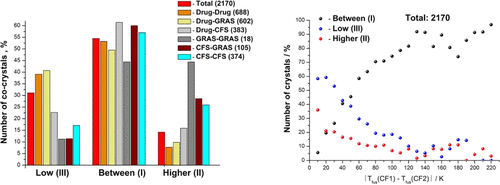
Based on literature analysis, we have built a database containing fusion temperatures and enthalpies of two-component molecular co-crystals and individual compounds (2170 co-crystals). The distribution functions of two-component crystals have been analyzed using their fusion temperatures, both for the database as a whole and separately for Drug–Drug, Drug–generally recognized as safe (GRAS), Drug–CF, and CF1–CF2 databases. A comparative analysis has been done of the co-crystal distribution by classes of compounds, I (“Between”), II (“Higher”), and III (“Low”), for the general database and six small databases. Correlation equations connecting the melting points of the co-crystals and individual components have been obtained, which has enabled us to design co-crystals with predictable melting temperatures. The effect of the difference between the melting temperatures of individual co-crystal components on the probability of formation of co-crystals of different groups (I–III) has been analyzed. An approach has been developed for evaluating the ideal solubility of co-crystals based on the knowledge of the ideal solubility of one of the co-crystal individual components only.
中文翻译:

双组分分子晶体:药物-药物、药物-GRAS 和 CF-CF 数据库之间的区别是什么?未知共晶的熔点和理想溶解度的评估
基于文献分析,我们建立了一个包含双组分分子共晶和单个化合物(2170个共晶)的熔化温度和焓的数据库。已经使用它们的融合温度分析了双组分晶体的分布函数,既针对整个数据库,也针对 Drug–Drug、Drug–generally公认为安全 (GRAS)、Drug–CF 和 CF1–CF2 数据库分别进行了分析。对于通用数据库和六个小型数据库,按化合物类别 I(“之间”)、II(“较高”)和 III(“低”)对共晶分布进行了比较分析。已经获得了连接共晶和单个组分的熔点的相关方程,这使我们能够设计具有可预测熔化温度的共晶。分析了单个共晶组分的熔化温度之间的差异对形成不同组 (I-III) 共晶的可能性的影响。已经开发了一种方法来评估共晶的理想溶解度,该方法仅基于共晶单个组分之一的理想溶解度的知识。
更新日期:2021-09-01
中文翻译:

双组分分子晶体:药物-药物、药物-GRAS 和 CF-CF 数据库之间的区别是什么?未知共晶的熔点和理想溶解度的评估
基于文献分析,我们建立了一个包含双组分分子共晶和单个化合物(2170个共晶)的熔化温度和焓的数据库。已经使用它们的融合温度分析了双组分晶体的分布函数,既针对整个数据库,也针对 Drug–Drug、Drug–generally公认为安全 (GRAS)、Drug–CF 和 CF1–CF2 数据库分别进行了分析。对于通用数据库和六个小型数据库,按化合物类别 I(“之间”)、II(“较高”)和 III(“低”)对共晶分布进行了比较分析。已经获得了连接共晶和单个组分的熔点的相关方程,这使我们能够设计具有可预测熔化温度的共晶。分析了单个共晶组分的熔化温度之间的差异对形成不同组 (I-III) 共晶的可能性的影响。已经开发了一种方法来评估共晶的理想溶解度,该方法仅基于共晶单个组分之一的理想溶解度的知识。



























 京公网安备 11010802027423号
京公网安备 11010802027423号