当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Copper(II)-Mediated Intermolecular Radical [3+2]-Annulation of N,N-Dimethyl Enaminones: Direct Access to 5-Acyl-3-Furancarboxaldehydes
Advanced Synthesis & Catalysis ( IF 5.4 ) Pub Date : 2021-07-26 , DOI: 10.1002/adsc.202100633 Biao Zhang 1 , Pan Zhou 1 , Hui Xu 1 , Jiuzhong Huang 2 , Yulin Sun 1 , Donghan Liu 1 , Fuchao Yu 1
Advanced Synthesis & Catalysis ( IF 5.4 ) Pub Date : 2021-07-26 , DOI: 10.1002/adsc.202100633 Biao Zhang 1 , Pan Zhou 1 , Hui Xu 1 , Jiuzhong Huang 2 , Yulin Sun 1 , Donghan Liu 1 , Fuchao Yu 1
Affiliation
|
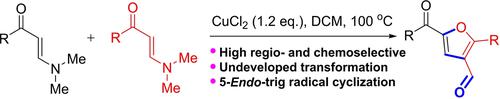
Copper(II)-mediated unprecedented intermolecular radical [3+2] annulation of N,N-dimethyl enaminones has been developed. The protocol is promoted simply by copper(II) chloride to access 5-acyl-3-furancarboxaldehydes with acceptable to good yields and broad substrate scope. This reaction allows the formation of multiple new bonds, including C(sp2)−O bond between two nucleophilic sites, C(sp2)−C(sp2) bond and C=O bond, through a radical cyclization process. Moreover, gram-synthesis and application research show the potential application value of this transformation in industry.
中文翻译:

铜(II)-介导的分子间自由基[3+2]-N,N-二甲基烯胺酮的环化:直接获得5-酰基-3-呋喃甲醛
铜 (II) 介导的前所未有的分子间自由基 [3+2] 环化N , N -二甲基烯胺酮已被开发出来。该协议仅由氯化铜 (II) 促进,以获取具有可接受的良好收率和广泛底物范围的 5-acyl-3-furancarboxaldehydes。该反应允许通过自由基环化过程形成多个新键,包括两个亲核位点之间的C( sp 2 )-O 键、C( sp 2 )-C( sp 2 ) 键和 C=O 键。此外,革兰氏合成和应用研究显示了这种转化在工业中的潜在应用价值。
更新日期:2021-09-21
中文翻译:

铜(II)-介导的分子间自由基[3+2]-N,N-二甲基烯胺酮的环化:直接获得5-酰基-3-呋喃甲醛
铜 (II) 介导的前所未有的分子间自由基 [3+2] 环化N , N -二甲基烯胺酮已被开发出来。该协议仅由氯化铜 (II) 促进,以获取具有可接受的良好收率和广泛底物范围的 5-acyl-3-furancarboxaldehydes。该反应允许通过自由基环化过程形成多个新键,包括两个亲核位点之间的C( sp 2 )-O 键、C( sp 2 )-C( sp 2 ) 键和 C=O 键。此外,革兰氏合成和应用研究显示了这种转化在工业中的潜在应用价值。



























 京公网安备 11010802027423号
京公网安备 11010802027423号