Sustainable Chemistry and Pharmacy ( IF 6 ) Pub Date : 2021-07-24 , DOI: 10.1016/j.scp.2021.100491 Prashant Mishra 1 , Kaman Singh 1 , Utkarsh Dixit 1
|
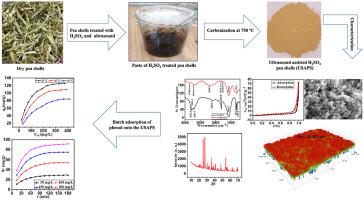
In the present study, waste pea shells were used to synthesize an efficient adsorbent (ultrasound-assisted sulphuric acid-treated pea shells, USAPS) and was applied for phenol removal. The USAPS characterization was done by SEM-EDS, FT-IR, XRD, optical profilometry, BET, and PZC techniques. The use of ultrasound during the chemical activation significantly enhanced the adsorption properties. The adsorption of phenol was probed by varying pH (2–9), temperature (25–45°C), the USAPS dose (0.1–0.6 g/100ml), phenol concentration (50–500 mg/L), and inorganic salt addition (0.1 M KCl and 0.1 M CaCl2). The maximum phenol uptake was found to be 125.77 mg/g for 500 mg/L of phenol concentration at pH 7 and 25°C with 0.1 g/100ml of the USAPS dose. Adsorption was negatively affected by an increase in temperature and the USAPS dose while 0.1 M KCl and 0.1 M CaCl2 addition decreased the maximum phenol uptake from 125.77 mg/g to 103.45 mg/g and 84.11 mg/g, respectively. The time-dependent phenol removal was best explained by the pseudo-second-order kinetic model while equilibrium data were best explained by the Langmuir model. The thermodynamic study revealed the physical nature of adsorption with no structural alteration at the adsorbent-adsorbate interface.
中文翻译:

超声波辅助硫酸处理豌豆(Pisum sativum)壳去除苯酚的吸附、动力学和热力学
在本研究中,废豌豆壳被用来合成一种高效的吸附剂(超声波辅助硫酸处理豌豆壳,USAPS),并用于去除苯酚。USAPS 表征是通过 SEM-EDS、FT-IR、XRD、光学轮廓测定法、BET 和 PZC 技术完成的。在化学活化过程中使用超声波显着增强了吸附性能。通过改变 pH (2-9)、温度 (25-45°C)、USAPS 剂量 (0.1-0.6 g/100ml)、苯酚浓度 (50-500 mg/L) 和无机盐来探测苯酚的吸附添加(0.1 M KCl 和 0.1 M CaCl 2)。在 pH 7 和 25°C 条件下,使用 0.1 g/100 ml USAPS 剂量,苯酚浓度为 500 mg/L 时,苯酚的最大吸收量为 125.77 mg/g。吸附受到温度升高和 USAPS 剂量的负面影响,而添加 0.1 M KCl 和 0.1 M CaCl 2将最大苯酚吸收分别从 125.77 mg/g 降低到 103.45 mg/g 和 84.11 mg/g。时间依赖的苯酚去除最好由伪二级动力学模型解释,而平衡数据最好由 Langmuir 模型解释。热力学研究揭示了吸附的物理性质,在吸附剂-被吸附物界面没有结构改变。



























 京公网安备 11010802027423号
京公网安备 11010802027423号