当前位置:
X-MOL 学术
›
Chin. J. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Bischler-Napieralski Cyclization: A Versatile Reaction towards Functional Aza-PAHs and Their Conjugated Polymers†
Chinese Journal of Chemistry ( IF 5.4 ) Pub Date : 2021-07-22 , DOI: 10.1002/cjoc.202100419 Mengwei Li 1 , Yuan Yuan 1 , Yulan Chen 1
Chinese Journal of Chemistry ( IF 5.4 ) Pub Date : 2021-07-22 , DOI: 10.1002/cjoc.202100419 Mengwei Li 1 , Yuan Yuan 1 , Yulan Chen 1
Affiliation
|
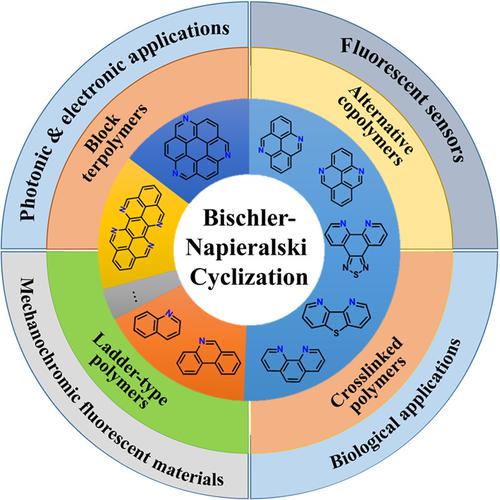
Nitrogen atom doped polycyclic aromatic hydrocarbons, the so-called aza-PAHs, and their conjugated polymers are applicable as functional chromophores and organic semiconductors. Prosperous developments of PAHs and heterocyclic chemistry have been made possible by considerable improvements in synthetic methodologies, among which one-step reactions leading directly to an aza-conjugated ring are highly desirable. Bischler- Napieralski cyclization, an old yet efficient reaction, can accomplish this task. During the last ten years, this reaction has been successfully used to synthesize a great variety of aza-PAHs molecules. Further combination with polymer chemistry is able to provide the corresponding polymers. This feature article summarizes the up-to-data advances, mainly achieved by our groups, on the design and synthesis of new and well-tailored aza-PAHs and macromolecular architectures using Bischler-Napieralski cyclization as the key step. Furthermore, the versatile applications of these functional materials in the fields of chemosensors, optoelectronic devices, and biomolecular probes are also covered in detail.
中文翻译:

Bischler-Napieralski 环化:对功能性 Aza-PAHs 及其共轭聚合物的多功能反应†
氮原子掺杂的多环芳烃,即所谓的氮杂多环芳烃及其共轭聚合物可用作功能发色团和有机半导体。PAHs 和杂环化学的蓬勃发展已经通过合成方法的显着改进成为可能,其中一步反应直接导致氮杂共轭环是非常可取的。Bischler-Napieralski 环化是一种古老而有效的反应,可以完成这项任务。在过去的十年中,该反应已成功用于合成多种氮杂多环芳烃分子。与聚合物化学的进一步结合能够提供相应的聚合物。这篇专题文章总结了最新的数据进展,主要是我们小组取得的,使用 Bischler-Napieralski 环化作为关键步骤设计和合成新的和精心定制的氮杂多环芳烃和大分子结构。此外,还详细介绍了这些功能材料在化学传感器、光电器件和生物分子探针领域的多功能应用。
更新日期:2021-07-22

中文翻译:

Bischler-Napieralski 环化:对功能性 Aza-PAHs 及其共轭聚合物的多功能反应†
氮原子掺杂的多环芳烃,即所谓的氮杂多环芳烃及其共轭聚合物可用作功能发色团和有机半导体。PAHs 和杂环化学的蓬勃发展已经通过合成方法的显着改进成为可能,其中一步反应直接导致氮杂共轭环是非常可取的。Bischler-Napieralski 环化是一种古老而有效的反应,可以完成这项任务。在过去的十年中,该反应已成功用于合成多种氮杂多环芳烃分子。与聚合物化学的进一步结合能够提供相应的聚合物。这篇专题文章总结了最新的数据进展,主要是我们小组取得的,使用 Bischler-Napieralski 环化作为关键步骤设计和合成新的和精心定制的氮杂多环芳烃和大分子结构。此外,还详细介绍了这些功能材料在化学传感器、光电器件和生物分子探针领域的多功能应用。




























 京公网安备 11010802027423号
京公网安备 11010802027423号