当前位置:
X-MOL 学术
›
J. Neurosci. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Sequential MAVS and MyD88/TRIF signaling triggers anti-viral responses of tick-borne encephalitis virus-infected murine astrocytes
Journal of Neuroscience Research ( IF 4.2 ) Pub Date : 2021-07-23 , DOI: 10.1002/jnr.24923 Luca Ghita 1 , Veronika Breitkopf 2 , Felix Mulenge 1 , Andreas Pavlou 1, 3 , Olivia Luise Gern 1, 4 , Verónica Durán 1 , Chittappen Kandiyil Prajeeth 3 , Moritz Kohls 5 , Klaus Jung 5 , Martin Stangel 3, 6 , Imke Steffen 2 , Ulrich Kalinke 1, 6
Journal of Neuroscience Research ( IF 4.2 ) Pub Date : 2021-07-23 , DOI: 10.1002/jnr.24923 Luca Ghita 1 , Veronika Breitkopf 2 , Felix Mulenge 1 , Andreas Pavlou 1, 3 , Olivia Luise Gern 1, 4 , Verónica Durán 1 , Chittappen Kandiyil Prajeeth 3 , Moritz Kohls 5 , Klaus Jung 5 , Martin Stangel 3, 6 , Imke Steffen 2 , Ulrich Kalinke 1, 6
Affiliation
|
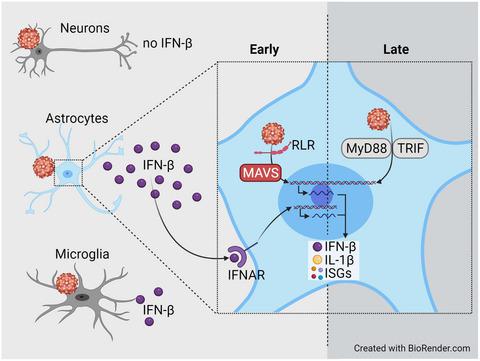
Tick-borne encephalitis virus (TBEV), a member of the Flaviviridae family, is typically transmitted upon tick bite and can cause meningitis and encephalitis in humans. In TBEV-infected mice, mitochondrial antiviral-signaling protein (MAVS), the downstream adaptor of retinoic acid-inducible gene-I (RIG-I)-like receptor (RLR) signaling, is needed to induce early type I interferon (IFN) responses and to confer protection. To characterize the brain-resident cell subset that produces protective IFN-β in TBEV-infected mice, we isolated neurons, astrocytes, and microglia from mice and exposed these cell types to TBEV in vitro. Under such conditions, neurons showed the highest percentage of infected cells, whereas astrocytes and microglia were infected to a lesser extent. In the supernatant (SN) of infected neurons, IFN-β was not detectable, while infected astrocytes showed high and microglia low IFN-β expression. Transcriptome analyses of astrocytes implied that MAVS signaling was needed early after TBEV infection. Accordingly, MAVS-deficient astrocytes showed enhanced TBEV infection and significantly reduced early IFN-β responses. Nevertheless, at later time points, moderate amounts of IFN-β were detected in the SN of infected MAVS-deficient astrocytes. Transcriptome analyses indicated that MAVS deficiency negatively affected the induction of early anti-viral responses, which resulted in significantly increased TBEV replication. Treatment with MyD88 and TRIF inhibiting peptides reduced only late IFN-β responses of TBEV-infected WT astrocytes and blocked entirely IFN-β responses of infected MAVS-deficient astrocytes. Thus, upon TBEV exposure of brain-resident cells, astrocytes are important IFN-β producers showing biphasic IFN-β induction that initially depends on MAVS and later on MyD88/TRIF signaling.
中文翻译:

连续的 MAVS 和 MyD88/TRIF 信号触发蜱传脑炎病毒感染的鼠星形胶质细胞的抗病毒反应
蜱传脑炎病毒 (TBEV),黄病毒科的成员家族,通常在蜱叮咬时传播,可导致人类脑膜炎和脑炎。在感染 TBEV 的小鼠中,需要线粒体抗病毒信号蛋白 (MAVS),即视黄酸诱导基因-I (RIG-I) 样受体 (RLR) 信号的下游接头来诱导早期 I 型干扰素 (IFN)回应并给予保护。为了表征在 TBEV 感染的小鼠中产生保护性 IFN-β 的大脑驻留细胞亚群,我们从小鼠中分离出神经元、星形胶质细胞和小胶质细胞,并将这些细胞类型在体外暴露于 TBEV。在这种情况下,神经元的感染细胞百分比最高,而星形胶质细胞和小胶质细胞的感染程度较低。在受感染神经元的上清液 (SN) 中,无法检测到 IFN-β,而受感染的星形胶质细胞则显示高表达和小胶质细胞低表达 IFN-β。星形胶质细胞的转录组分析暗示在 TBEV 感染后早期需要 MAVS 信号传导。因此,缺乏 MAVS 的星形胶质细胞表现出增强的 TBEV 感染并显着降低了早期 IFN-β 反应。然而,在后来的时间点,在感染的 MAVS 缺陷型星形胶质细胞的 SN 中检测到适量的 IFN-β。转录组分析表明,MAVS 缺乏对早期抗病毒反应的诱导产生负面影响,导致 TBEV 复制显着增加。用 MyD88 和 TRIF 抑制肽治疗仅减少 TBEV 感染的 WT 星形胶质细胞的晚期 IFN-β 反应,并完全阻断感染的 MAVS 缺陷型星形胶质细胞的 IFN-β 反应。因此,在大脑驻留细胞暴露于 TBEV 后,
更新日期:2021-07-23
中文翻译:

连续的 MAVS 和 MyD88/TRIF 信号触发蜱传脑炎病毒感染的鼠星形胶质细胞的抗病毒反应
蜱传脑炎病毒 (TBEV),黄病毒科的成员家族,通常在蜱叮咬时传播,可导致人类脑膜炎和脑炎。在感染 TBEV 的小鼠中,需要线粒体抗病毒信号蛋白 (MAVS),即视黄酸诱导基因-I (RIG-I) 样受体 (RLR) 信号的下游接头来诱导早期 I 型干扰素 (IFN)回应并给予保护。为了表征在 TBEV 感染的小鼠中产生保护性 IFN-β 的大脑驻留细胞亚群,我们从小鼠中分离出神经元、星形胶质细胞和小胶质细胞,并将这些细胞类型在体外暴露于 TBEV。在这种情况下,神经元的感染细胞百分比最高,而星形胶质细胞和小胶质细胞的感染程度较低。在受感染神经元的上清液 (SN) 中,无法检测到 IFN-β,而受感染的星形胶质细胞则显示高表达和小胶质细胞低表达 IFN-β。星形胶质细胞的转录组分析暗示在 TBEV 感染后早期需要 MAVS 信号传导。因此,缺乏 MAVS 的星形胶质细胞表现出增强的 TBEV 感染并显着降低了早期 IFN-β 反应。然而,在后来的时间点,在感染的 MAVS 缺陷型星形胶质细胞的 SN 中检测到适量的 IFN-β。转录组分析表明,MAVS 缺乏对早期抗病毒反应的诱导产生负面影响,导致 TBEV 复制显着增加。用 MyD88 和 TRIF 抑制肽治疗仅减少 TBEV 感染的 WT 星形胶质细胞的晚期 IFN-β 反应,并完全阻断感染的 MAVS 缺陷型星形胶质细胞的 IFN-β 反应。因此,在大脑驻留细胞暴露于 TBEV 后,



























 京公网安备 11010802027423号
京公网安备 11010802027423号