Journal of Organometallic Chemistry ( IF 2.3 ) Pub Date : 2021-07-23 , DOI: 10.1016/j.jorganchem.2021.122002 Sarvarbek Salohiddinov 1, 2 , Arumugam Vignesh 1 , Zilong Li 1, 2 , Yanping Ma 1, 2 , Wen-Hua Sun 1, 2
|
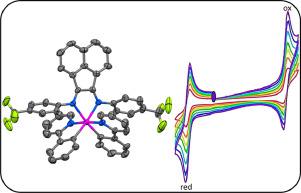
Three cationic iridium (III) complexes, [(ppy)2Ir(4-FPhBIAN)]PF6 (Ir1), [(ppy)2Ir(3,4-F,FPhBIAN)]PF6 (Ir2), [(ppy)2Ir(4-CF3PhBIAN)]PF6 (Ir3) were synthesized from 2-phenylpyridine (Hppy) as cyclometalating ligand and N,N′-bis(aryl)acenaphthene as ancillary ligand. All the complexes were characterized by FT-IR, 1H, 13C and 19F NMR spectroscopies, and elemental analysis. The molecular structures of the complexes were determined by X-ray crystallography. These complexes were crystalized as greenish-black needles in the triclinic P1 space group for complexes Ir1 and Ir3, while Ir2 was crystalized in the orthorhombic P212121 space group. Electrochemical properties were studied by cyclic voltammetry analysis. All the complexes showed quasi-reversible oxidation and reduction peaks, and reversibility increased by scan rate. Electronic properties were studied by UV-visible spectroscopy, and intense absorption band below 300 nm was obtained compared to intense absorption bands between 300 ~ 530 nm. Density functional theory (DFT) calculations were employed to get better understanding of electrochemical properties.
中文翻译:

带有氟化 Ar-BIAN 配体的阳离子铱 (III) 配合物:合成、结构、电子和电化学特性。
三种阳离子铱(III)络合物,[(PPY)2的Ir(4-FPhBIAN)] PF 6(IR1),[(PPY)2的Ir(3,4--F,FPhBIAN)] PF 6(亮点Ir2),[( ppy) 2 Ir(4-CF 3 PhBIAN)]PF 6 ( Ir3 ) 由 2-苯基吡啶 (Hppy) 作为环金属化配体和 N,N'-双(芳基)苊作为辅助配体合成。所有配合物均通过 FT-IR、1 H、13 C 和19F NMR光谱和元素分析。配合物的分子结构由 X 射线晶体学确定。这些配合物在配合物Ir1和Ir3的三斜P1空间群中结晶为绿黑色针状,而Ir2在正交P2 1 2 1 2 1 中结晶空间群。通过循环伏安法分析来研究电化学性质。所有配合物均表现出准可逆的氧化和还原峰,可逆性随扫描速率的增加而增加。通过紫外-可见光谱研究了电子特性,与300~530 nm之间的强吸收带相比,获得了300 nm以下的强吸收带。采用密度泛函理论 (DFT) 计算来更好地了解电化学性质。


























 京公网安备 11010802027423号
京公网安备 11010802027423号