当前位置:
X-MOL 学术
›
Chem. Eur. J.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Determining the Diastereoselectivity of the Formation of Dipeptidonucleotides by NMR Spectroscopy
Chemistry - A European Journal ( IF 4.3 ) Pub Date : 2021-07-22 , DOI: 10.1002/chem.202101630 Olivia Doppleb 1 , Jennifer Bremer 1 , Maren Bechthold 1 , Carolina Sánchez Rico 1 , Daniela Göhringer 1 , Helmut Griesser 1 , Clemens Richert 1
Chemistry - A European Journal ( IF 4.3 ) Pub Date : 2021-07-22 , DOI: 10.1002/chem.202101630 Olivia Doppleb 1 , Jennifer Bremer 1 , Maren Bechthold 1 , Carolina Sánchez Rico 1 , Daniela Göhringer 1 , Helmut Griesser 1 , Clemens Richert 1
Affiliation
|
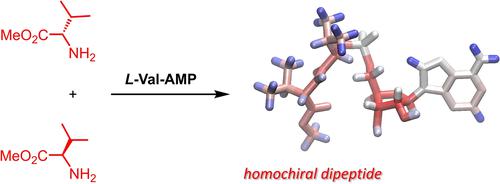
Proteins are composed of l-amino acids, but nucleic acids and most oligosaccharides contain d-sugars as building blocks. It is interesting to ask whether this is a coincidence or a consequence of the functional interplay of these biomolecules. One reaction that provides an opportunity to study this interplay is the formation of phosphoramidate-linked peptido RNA from amino acids and ribonucleotides in aqueous condensation buffer. Here we report how the diastereoselectivity of the first peptide coupling of the peptido RNA pathway can be determined in situ by NMR spectroscopy. When a racemic mixture of an amino acid ester was allowed to react with an 5′-aminoacidyl nucleotide, diastereomeric ratios of up to 72 : 28 of the resulting dipeptido nucleotides were found by integration of 31P- or 1H-NMR peaks. The highest diastereomeric excess was found for the homochiral coupling product d-Ser-d-Trp, phosphoramidate-linked to adenosine 5′-monophosphate with its d-ribose ring. When control reactions with an N-acetyl amino acid and valine methyl ester were run in organic solvent, the diastereoselectivity was found to be lower, with diastereomeric ratios≤62 : 38. The results from the exploratory study thus indicate that the ribonucleotide residue not only facilitates the coupling of lipophilic amino acids in aqueous medium but also the formation of a homochiral dipeptide. The methodology described here may be used to search for other stereoselective reactions that shed light on the origin of homochirality.
中文翻译:

用核磁共振光谱法测定二肽核苷酸形成的非对映选择性
蛋白质由l-氨基酸组成,但核酸和大多数寡糖含有d-糖作为构建块。有趣的是要问这是巧合还是这些生物分子功能相互作用的结果。为研究这种相互作用提供机会的一个反应是在水性缩合缓冲液中由氨基酸和核糖核苷酸形成氨基磷酸酯连接的肽 RNA。在这里,我们报告了如何通过 NMR 光谱原位确定肽 RNA 途径的第一个肽偶联的非对映选择性。当氨基酸酯的外消旋混合物与 5'-氨基酸基核苷酸反应时,通过整合31 P-或1 H-NMR峰。发现同手性偶联产物d -Ser - d -Trp 的非对映体过量最高,氨基磷酸酯通过其d-核糖环与腺苷 5'-单磷酸相连。当用N控制反应时-乙酰氨基酸和缬氨酸甲酯在有机溶剂中运行,发现非对映选择性较低,非对映比≤62:38。因此探索性研究的结果表明,核糖核苷酸残基不仅促进了亲脂性氨基的偶联水介质中的酸,但也形成同手性二肽。此处描述的方法可用于搜索揭示同手性起源的其他立体选择性反应。
更新日期:2021-09-24
中文翻译:

用核磁共振光谱法测定二肽核苷酸形成的非对映选择性
蛋白质由l-氨基酸组成,但核酸和大多数寡糖含有d-糖作为构建块。有趣的是要问这是巧合还是这些生物分子功能相互作用的结果。为研究这种相互作用提供机会的一个反应是在水性缩合缓冲液中由氨基酸和核糖核苷酸形成氨基磷酸酯连接的肽 RNA。在这里,我们报告了如何通过 NMR 光谱原位确定肽 RNA 途径的第一个肽偶联的非对映选择性。当氨基酸酯的外消旋混合物与 5'-氨基酸基核苷酸反应时,通过整合31 P-或1 H-NMR峰。发现同手性偶联产物d -Ser - d -Trp 的非对映体过量最高,氨基磷酸酯通过其d-核糖环与腺苷 5'-单磷酸相连。当用N控制反应时-乙酰氨基酸和缬氨酸甲酯在有机溶剂中运行,发现非对映选择性较低,非对映比≤62:38。因此探索性研究的结果表明,核糖核苷酸残基不仅促进了亲脂性氨基的偶联水介质中的酸,但也形成同手性二肽。此处描述的方法可用于搜索揭示同手性起源的其他立体选择性反应。



























 京公网安备 11010802027423号
京公网安备 11010802027423号