Cell Host & Microbe ( IF 30.3 ) Pub Date : 2021-07-21 , DOI: 10.1016/j.chom.2021.06.014 Xue-Song Zhang 1 , Yue Sandra Yin 1 , Jincheng Wang 2 , Thomas Battaglia 3 , Kimberly Krautkramer 4 , Wei Vivian Li 5 , Jackie Li 3 , Mark Brown 6 , Meifan Zhang 1 , Michelle H Badri 7 , Abigail J S Armstrong 8 , Christopher M Strauch 9 , Zeneng Wang 9 , Ina Nemet 9 , Nicole Altomare 8 , Joseph C Devlin 3 , Linchen He 10 , Jamie T Morton 11 , John Alex Chalk 8 , Kelly Needles 8 , Viviane Liao 8 , Julia Mount 3 , Huilin Li 10 , Kelly V Ruggles 3 , Richard A Bonneau 12 , Maria Gloria Dominguez-Bello 13 , Fredrik Bäckhed 14 , Stanley L Hazen 15 , Martin J Blaser 1
|
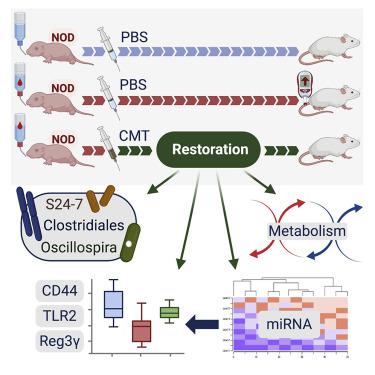
Early-life antibiotic exposure perturbs the intestinal microbiota and accelerates type 1 diabetes (T1D) development in the NOD mouse model. Here, we found that maternal cecal microbiota transfer (CMT) to NOD mice after early-life antibiotic perturbation largely rescued the induced T1D enhancement. Restoration of the intestinal microbiome was significant and persistent, remediating the antibiotic-depleted diversity, relative abundance of particular taxa, and metabolic pathways. CMT also protected against perturbed metabolites and normalized innate and adaptive immune effectors. CMT restored major patterns of ileal microRNA and histone regulation of gene expression. Further experiments suggest a gut-microbiota-regulated T1D protection mechanism centered on Reg3γ, in an innate intestinal immune network involving CD44, TLR2, and Reg3γ. This regulation affects downstream immunological tone, which may lead to protection against tissue-specific T1D injury.
中文翻译:

母体盲肠微生物群转移可挽救早期抗生素诱导的小鼠 1 型糖尿病增强
在 NOD 小鼠模型中,早期接触抗生素会扰乱肠道微生物群并加速 1 型糖尿病 (T1D) 的发展。在这里,我们发现在早期抗生素扰动后母体盲肠微生物群转移 (CMT) 到 NOD 小鼠在很大程度上挽救了诱导的 T1D 增强。肠道微生物组的恢复是显着且持久的,修复了抗生素耗尽的多样性、特定类群的相对丰度和代谢途径。CMT 还可以防止代谢物紊乱,并使先天和适应性免疫效应器正常化。CMT 恢复了回肠 microRNA 的主要模式和基因表达的组蛋白调控。进一步的实验表明,在涉及 CD44、TLR2 和 Reg3γ 的先天性肠道免疫网络中,肠道微生物群调节的 T1D 保护机制以 Reg3γ 为中心。



























 京公网安备 11010802027423号
京公网安备 11010802027423号