Journal of Inorganic Biochemistry ( IF 3.9 ) Pub Date : 2021-07-21 , DOI: 10.1016/j.jinorgbio.2021.111556 Farideh Jalilehvand 1 , Alejandra Enriquez Garcia 1 , Pantea Niksirat 1 , Y Zou Finfrock 2 , Benjamin S Gelfand 1
|
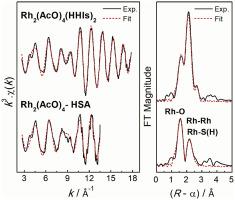
Reactions of the anticancer active dirhodium tetraacetate (1), Rh2(AcO)4 (AcO− = CH3COO−), with the amino acid histidine (HHis) and human serum albumin (HSA) were monitored over time and different metal: ligand ratios using UV-vis spectroscopy and/or electro-spray ionization mass spectrometry. Initially, histidine formed 1:1 and 1:2 adducts in aqueous solutions. The crystal structure of Rh2(AcO)4(L-HHis)2·2H2O (2) confirmed the axial coordination of histidine imidazole groups (average Rh-Naxial 2.23 Å). These adducts, however, were found to be unstable in solution over time (24 h). Heating Rh2(AcO)4 –histidine solutions to 40 °C (near body temperature) or 95 °C accelerated the formation of RhII2(AcO)2(His)2 and RhIII(His)2(AcO) complexes. The corresponding pH change from neutral to mildly acid (pH 4–5) indicates deprotonation of histidine NH3+ groups due to coordination to Rh ions, which simultaneously bind to histidine COO− groups, as evidenced by 13C NMR spectroscopy. In the case of HSA with 16 histidine and one cysteine residues, UV-vis spectroscopy indicates that mono- and di-histidine HSA adducts with Rh2(AcO)4 are formed. X-ray absorption spectroscopy showed almost the same Rh-Rh distance (2.41 ± 0.01 Å) for the Rh2(AcO)4 units as in 2, and a contribution from an axial thiol coordination (Rh-Saxial 2.62 ± 0.05 Å). The Rh2(AcO)4 – HSA complex was found to decompose partially (~15%) over 24 h at ambient temperature. The partial decomposition of Rh2(AcO)4 both through coordination to histidine or to human serum albumin, the most abundant protein in blood plasma, is a factor to consider for its efficacy as a potential anticancer agent.
中文翻译:

组氨酸和人血清白蛋白与四乙酸二铑(II)的结合
随着时间的推移和不同的金属监测抗癌活性四乙酸二铑 ( 1 )、Rh 2 (AcO) 4 (AcO - = CH 3 COO - ) 与氨基酸组氨酸 (HHis) 和人血清白蛋白 (HSA) 的反应:使用紫外-可见光谱法和/或电喷雾电离质谱法测定配体比率。最初,组氨酸在水溶液中形成 1:1 和 1:2 的加合物。Rh 2 (AcO) 4 ( L -HHis) 2 · 2H 2 O ( 2 )的晶体结构证实了组氨酸咪唑基团的轴向配位(平均Rh-N轴向2.23 埃)。然而,发现这些加合物在溶液中随时间(24 小时)不稳定。将Rh 2 (AcO) 4 -组氨酸溶液加热到 40 °C(接近体温)或 95 °C 加速了 Rh II 2 (AcO) 2 (His) 2和 Rh III (His) 2 (AcO) 复合物的形成。相应的 pH 从中性变为弱酸性(pH 4-5)表明组氨酸 NH 3 +基团由于与 Rh 离子的配位而去质子化,同时与组氨酸 COO -基团结合,如13所示C 核磁共振波谱。在具有 16 个组氨酸和一个半胱氨酸残基的 HSA 的情况下,UV-vis 光谱表明形成了具有 Rh 2 (AcO) 4的单组氨酸和二组氨酸 HSA 加合物。X 射线吸收光谱显示 Rh 2 (AcO) 4单元的 Rh-Rh 距离 (2.41 ± 0.01 Å) 与2中的几乎相同,并且来自轴向硫醇配位 (Rh-S轴向2.62 ± 0.05 Å) . 发现Rh 2 (AcO) 4 – HSA 复合物在环境温度下在 24 小时内部分分解(~15%)。Rh 2 (AcO) 4的部分分解通过与组氨酸或人血清白蛋白(血浆中最丰富的蛋白质)的配位,是考虑其作为潜在抗癌剂功效的一个因素。


























 京公网安备 11010802027423号
京公网安备 11010802027423号