Journal of Advanced Research ( IF 10.7 ) Pub Date : 2021-07-22 , DOI: 10.1016/j.jare.2021.07.007 Vijayakumar Jawalagatti 1 , Perumalraja Kirthika 1 , Ji-Young Park 1 , Chamith Hewawaduge 1 , John Hwa Lee 1
|
Introduction
The emergence of SARS-CoV-2 variants has raised concerns on future vaccine efficacy as most vaccines target only the spike protein. Hence, vaccines targeting multiple SARS-CoV-2 proteins will offer broader protection and improve our preparedness to combat the pandemic.
Objectives
The study aimed to develop a novel vaccine strategy by combining a eukaryotic vector expressing multiple SARS-CoV-2 genes and Salmonella-mediated in vivo DNA delivery.
Methods
The eukaryotic vector was designed to function as a DNA-launched RNA replicon in a self-replicating and self-amplifying mRNA mechanism. By exploiting the self-cleaving peptide, P2A, we fused four SARS-CoV-2 targets, including receptor-binding domain (RBD), heptad repeat domain (HR), membrane protein (M) and epitopes of nsp13, in a single open reading frame. Western blot and immunofluorescence assays were used to determine protein expression. In mice, the vaccine's safety and immunogenicity were investigated.
Results
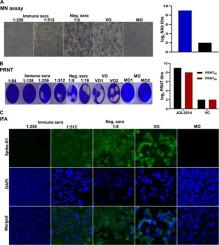
Western blot analysis revealed co-expression all four proteins from the vaccine construct, confirming the efficiency of Salmonella-mediated gene delivery and protein expression. The vaccine candidate was safe and elicited robust antigen-specific antibody titers in mice, and a recall response from splenocytes revealed induction of strong cell-mediated immunity. Flow cytometry demonstrated an increase in sub-populations of CD4+ and CD8+ T cells with the highest CD4+ and CD8+ T cells recorded for HR and RBD, respectively. Overall, humoral and cellular immune response data suggested the induction of both Th1 and Th2 immunity with polarization towards an antiviral Th1 response. We recorded a potent SARS-CoV-2 neutralizing antibody titers in the immunized mice sera.
Conclusions
The Salmonella bactofection ensured optimum in vivo gene delivery, and through a P2A-enabled efficient multicistronic expression, the vaccine candidate elicited potent anti-SARS-CoV-2 immune responses. These findings provide important insight into development of an effective multivalent vaccine to combat SARS-CoV-2 and its variants.
中文翻译:

高度可行的免疫保护性多顺反子 SARS-CoV-2 候选疫苗,融合了新型真核表达和沙门氏菌细菌感染
介绍
SARS-CoV-2 变体的出现引发了人们对未来疫苗功效的担忧,因为大多数疫苗仅针对刺突蛋白。因此,针对多种 SARS-CoV-2 蛋白的疫苗将提供更广泛的保护,并提高我们抗击这一流行病的准备。
目标
该研究旨在通过结合表达多个 SARS-CoV-2 基因的真核载体和沙门氏菌介导的体内DNA 递送来开发一种新的疫苗策略。
方法
真核载体被设计为在自我复制和自我放大的 mRNA 机制中充当 DNA 启动的 RNA 复制子。通过利用自切割肽 P2A,我们将四个 SARS-CoV-2 靶标融合在一起,包括受体结合域 (RBD)、七肽重复域 (HR)、膜蛋白 (M) 和 nsp13 的表位。阅读框架。蛋白质印迹和免疫荧光测定用于确定蛋白质表达。在小鼠中,研究了疫苗的安全性和免疫原性。
结果
蛋白质印迹分析揭示了来自疫苗构建体的所有四种蛋白质的共表达,证实了沙门氏菌介导的基因递送和蛋白质表达的效率。候选疫苗是安全的,并在小鼠体内引发了强大的抗原特异性抗体滴度,脾细胞的回忆反应显示诱导了强细胞介导的免疫。流式细胞术显示 CD4 +和 CD8 + T 细胞亚群增加,具有最高的 CD4 +和 CD8 +分别记录 HR 和 RBD 的 T 细胞。总体而言,体液和细胞免疫反应数据表明 Th1 和 Th2 免疫的诱导与抗病毒 Th1 反应的极化。我们在免疫小鼠血清中记录了有效的 SARS-CoV-2 中和抗体滴度。
结论
沙门氏菌转染确保了最佳的体内基因传递,并且通过 P2A 启用的有效多顺反子表达,候选疫苗引发了有效的抗 SARS-CoV-2 免疫反应。这些发现为开发有效的多价疫苗以对抗 SARS-CoV-2 及其变体提供了重要的见解。



























 京公网安备 11010802027423号
京公网安备 11010802027423号