当前位置:
X-MOL 学术
›
ACS Pharmacol. Transl. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Structure-Based Design of First-Generation Small Molecule Inhibitors Targeting the Catalytic Pockets of AID, APOBEC3A, and APOBEC3B
ACS Pharmacology & Translational Science Pub Date : 2021-07-19 , DOI: 10.1021/acsptsci.1c00091 Justin J King 1, 2 , Faezeh Borzooee 1, 2 , Junbum Im 2, 3 , Mahdi Asgharpour 1, 2 , Atefeh Ghorbani 1, 2 , Cody P Diamond 2 , Heather Fifield 2 , Lesley Berghuis 2 , Mani Larijani 1, 2
ACS Pharmacology & Translational Science Pub Date : 2021-07-19 , DOI: 10.1021/acsptsci.1c00091 Justin J King 1, 2 , Faezeh Borzooee 1, 2 , Junbum Im 2, 3 , Mahdi Asgharpour 1, 2 , Atefeh Ghorbani 1, 2 , Cody P Diamond 2 , Heather Fifield 2 , Lesley Berghuis 2 , Mani Larijani 1, 2
Affiliation
|
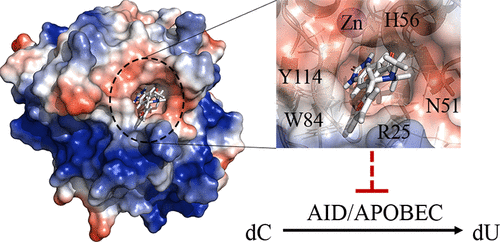
Activation-induced cytidine deaminase (AID) initiates antibody diversification by mutating immunoglobulin loci in B lymphocytes. AID and related APOBEC3 (A3) enzymes also induce genome-wide mutations and lesions implicated in tumorigenesis and tumor progression. The most prevalent mutation signatures across diverse tumor genomes are attributable to the mistargeted mutagenic activities of AID/A3s. Thus, inhibiting AID/A3s has been suggested to be of therapeutic benefit. We previously used a computational-biochemical approach to gain insight into the structure of AID’s catalytic pocket, which resulted in the discovery of a novel type of regulatory catalytic pocket closure that regulates AID/A3s that we termed the “Schrodinger’s CATalytic pocket”. Our findings were subsequently confirmed by direct structural studies. Here, we describe our search for small molecules that target the catalytic pocket of AID. We identified small molecules that inhibit purified AID, AID in cell extracts, and endogenous AID of lymphoma cells. Analogue expansion yielded derivatives with improved potencies. These were found to also inhibit A3A and A3B, the two most tumorigenic siblings of AID. Two compounds exhibit low micromolar IC50 inhibition of AID and A3A, exhibiting the strongest potency for A3A. Docking suggests key interactions between their warheads and residues lining the catalytic pockets of AID, A3A, and A3B and between the tails and DNA-interacting residues on the surface proximal to the catalytic pocket opening. Accordingly, mutants of these residues decreased inhibition potency. The chemistry and abundance of key stabilizing interactions between the small molecules and residues within and immediately outside the catalytic pockets are promising for therapeutic development.
中文翻译:

针对 AID、APOBEC3A 和 APOBEC3B 催化口袋的第一代小分子抑制剂的基于结构的设计
活化诱导的胞苷脱氨酶 (AID) 通过突变 B 淋巴细胞中的免疫球蛋白基因座来启动抗体多样化。AID 和相关的 APOBEC3 (A3) 酶也诱导与肿瘤发生和肿瘤进展有关的全基因组突变和损伤。不同肿瘤基因组中最普遍的突变特征可归因于 AID/A3 的错误靶向诱变活性。因此,抑制 AID/A3s 被认为具有治疗益处。我们之前使用计算生化方法来深入了解 AID 催化口袋的结构,从而发现了一种新型的调节 AID/A3 的调节性催化口袋闭合,我们称之为“薛定谔的催化口袋”。我们的发现随后通过直接结构研究得到证实。这里,我们描述了我们对针对 AID 催化口袋的小分子的搜索。我们鉴定了抑制纯化的 AID、细胞提取物中的 AID 和淋巴瘤细胞的内源性 AID 的小分子。类似的扩展产生了具有改进效力的衍生物。发现这些也抑制 A3A 和 A3B,这是 AID 的两个最易致瘤的兄弟姐妹。两种化合物表现出低微摩尔 IC50抑制 AID 和 A3A,对 A3A 表现出最强的效力。对接表明它们的弹头与 AID、A3A 和 A3B 催化袋内的残留物之间以及尾部与靠近催化袋开口的表面上的 DNA 相互作用残留物之间存在关键相互作用。因此,这些残基的突变体降低了抑制效力。小分子与催化袋内部和外部的残基之间的关键稳定相互作用的化学性质和丰富程度有望用于治疗开发。
更新日期:2021-08-13
中文翻译:

针对 AID、APOBEC3A 和 APOBEC3B 催化口袋的第一代小分子抑制剂的基于结构的设计
活化诱导的胞苷脱氨酶 (AID) 通过突变 B 淋巴细胞中的免疫球蛋白基因座来启动抗体多样化。AID 和相关的 APOBEC3 (A3) 酶也诱导与肿瘤发生和肿瘤进展有关的全基因组突变和损伤。不同肿瘤基因组中最普遍的突变特征可归因于 AID/A3 的错误靶向诱变活性。因此,抑制 AID/A3s 被认为具有治疗益处。我们之前使用计算生化方法来深入了解 AID 催化口袋的结构,从而发现了一种新型的调节 AID/A3 的调节性催化口袋闭合,我们称之为“薛定谔的催化口袋”。我们的发现随后通过直接结构研究得到证实。这里,我们描述了我们对针对 AID 催化口袋的小分子的搜索。我们鉴定了抑制纯化的 AID、细胞提取物中的 AID 和淋巴瘤细胞的内源性 AID 的小分子。类似的扩展产生了具有改进效力的衍生物。发现这些也抑制 A3A 和 A3B,这是 AID 的两个最易致瘤的兄弟姐妹。两种化合物表现出低微摩尔 IC50抑制 AID 和 A3A,对 A3A 表现出最强的效力。对接表明它们的弹头与 AID、A3A 和 A3B 催化袋内的残留物之间以及尾部与靠近催化袋开口的表面上的 DNA 相互作用残留物之间存在关键相互作用。因此,这些残基的突变体降低了抑制效力。小分子与催化袋内部和外部的残基之间的关键稳定相互作用的化学性质和丰富程度有望用于治疗开发。



























 京公网安备 11010802027423号
京公网安备 11010802027423号