Immunity ( IF 32.4 ) Pub Date : 2021-07-20 , DOI: 10.1016/j.immuni.2021.07.002 Daniel Hofbauer 1 , Dimitrios Mougiakakos 1 , Luca Broggini 2 , Mario Zaiss 3 , Maike Büttner-Herold 4 , Christian Bach 1 , Bernd Spriewald 1 , Frank Neumann 5 , Savita Bisht 6 , Jens Nolting 6 , Robert Zeiser 7 , Shaima'a Hamarsheh 7 , Martin Eberhardt 8 , Julio Vera 8 , Cristina Visentin 9 , Chiara Maria Giulia De Luca 10 , Fabio Moda 10 , Stefan Haskamp 11 , Cindy Flamann 1 , Martin Böttcher 1 , Katrin Bitterer 1 , Simon Völkl 1 , Andreas Mackensen 1 , Stefano Ricagno 9 , Heiko Bruns 1
|
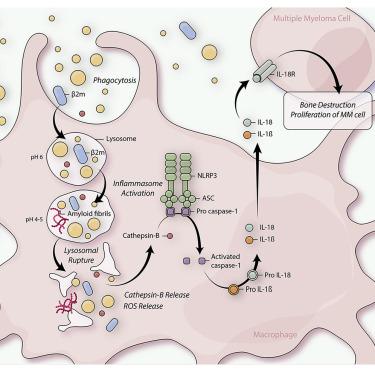
As substantial constituents of the multiple myeloma (MM) microenvironment, pro-inflammatory macrophages have emerged as key promoters of disease progression, bone destruction, and immune impairment. We identify beta-2-microglobulin (β2m) as a driver in initiating inflammation in myeloma-associated macrophages (MAMs). Lysosomal accumulation of phagocytosed β2m promotes β2m amyloid aggregation in MAMs, resulting in lysosomal rupture and ultimately production of active interleukin-1β (IL-1β) and IL-18. This process depends on activation of the NLRP3 inflammasome after β2m accumulation, as macrophages from NLRP3-deficient mice lack efficient β2m-induced IL-1β production. Moreover, depletion or silencing of β2m in MM cells abrogates inflammasome activation in a murine MM model. Finally, we demonstrate that disruption of NLRP3 or IL-18 diminishes tumor growth and osteolytic bone destruction normally promoted by β2m-induced inflammasome signaling. Our results provide mechanistic evidence for β2m’s role as an NLRP3 inflammasome activator during MM pathogenesis. Moreover, inhibition of NLRP3 represents a potential therapeutic approach in MM.
中文翻译:

β2-微球蛋白在肿瘤相关巨噬细胞中触发 NLRP3 炎性体激活以促进多发性骨髓瘤进展
作为多发性骨髓瘤 (MM) 微环境的重要组成部分,促炎巨噬细胞已成为疾病进展、骨骼破坏和免疫损伤的关键促进因素。我们将 β-2-微球蛋白 (β2m) 确定为引发骨髓瘤相关巨噬细胞 (MAM) 炎症的驱动因素。吞噬的 β2m 的溶酶体积累促进 MAM 中 β2m 淀粉样蛋白的聚集,导致溶酶体破裂并最终产生活性白细胞介素-1β (IL-1β) 和 IL-18。这个过程取决于 β2m 积累后 NLRP3 炎症小体的激活,因为来自 NLRP3 缺陷小鼠的巨噬细胞缺乏有效的 β2m 诱导的 IL-1β 产生。此外,MM 细胞中 β2m 的消耗或沉默消除了小鼠 MM 模型中的炎症小体激活。最后,我们证明 NLRP3 或 IL-18 的破坏减少了通常由 β2m 诱导的炎性体信号传导促进的肿瘤生长和溶骨性骨破坏。我们的结果为 β2m 在 MM 发病过程中作为 NLRP3 炎症小体激活剂的作用提供了机制证据。此外,抑制 NLRP3 代表了 MM 的潜在治疗方法。


























 京公网安备 11010802027423号
京公网安备 11010802027423号