Water Research ( IF 12.8 ) Pub Date : 2021-07-18 , DOI: 10.1016/j.watres.2021.117445 Huaying Liu 1 , Zhichao Hou 1 , Yingjie Li 1 , Yajie Lei 1 , Zihao Xu 1 , Junjie Gu 1 , Senlin Tian 1
|
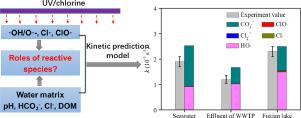
The UV/chlorine system has been regarded as an efficient oxidation technology for the removal of aqueous micropollutants. However, the roles of the possible radical species for this system on the elimination under environmentally relevant conditions/real waters were still largely unknown. Herein, the specific roles of radical species in the UV/chlorine oxidation degradation of gemfibrozil and naproxen as representative micropollutants were quantified by a steady-state kinetic prediction model considering the effects of water matrices. Overall, the model predicted results are consistent with the experimental data well. •OH and reactive chlorine species (RCS, such as Cl•, ClO•, and Cl2•−) contributions to gemfibrozil and naproxen degradation were water matrix specific. In pure water, both primary reactive species (i.e., •OH and Cl•) and secondary species ClO• dominated gemfibrozil and naproxen degradation, and their individual and the sum of the contributions to degradation rates reduced with pH increase of from 5 to 9. In the presence of Cl−, we found that Cl2•− and in particular ClO• were responsible for the enhanced degradation with increasing Cl− concentrations due to the considerable ClO• reactivity of gemfibrozil (1.93 × 109 M−1 s−1) and naproxen (9.24 × 109 M−1 s−1) and the rapid transformation of Cl2•− to ClO•. The presence of HCO3− notably facilitated the degradation in the UV/chlorine process because of the generation of CO3•−. CO3•− showed high reactivity with gemfibrozil and naproxen corresponding to respective second-order reaction rate constants of 2.45 × 107 and 3.50 × 107 M−1 s−1. Dissolved organic matter induced obvious scavenging for •OH, Cl•, and ClO• and greatly retarded the degradation. The constructed model considering the effects of above water matrix has successfully predicted the oxidation degradation kinetics in real waters, and both •OH and CO3•− are the predominant reactive species in the degradation. This study is helpful for comprehensive understanding the roles of possible radical species in micropollutant removal by UV/chlorine oxidation under real water matrix.
中文翻译:

紫外线/氯系统中吉非贝齐和萘普生的降解动力学建模:活性物质的作用和水基质的影响
紫外线/氯系统被认为是去除水性微污染物的有效氧化技术。然而,该系统可能的自由基物种在环境相关条件/真实水域中的消除作用仍然很大程度上未知。在此,通过考虑水基质影响的稳态动力学预测模型量化了自由基物种在作为代表性微污染物的吉非贝齐和萘普生的紫外线/氯氧化降解中的具体作用。总体而言,模型预测结果与实验数据吻合较好。•OH 和活性氯物质(RCS,例如 Cl•、ClO• 和 Cl 2 • -) 对吉非罗齐和萘普生降解的贡献是水基质特异性的。在纯水中,主要活性物质(即 •OH 和 Cl•)和次要物质 ClO• 主导吉非贝齐和萘普生的降解,并且它们的个体和对降解速率的贡献的总和随着 pH 从 5 增加到 9 而降低。在氯的存在下- ,我们发现,氯2 • -并且特别CLO•负责随氯增强降解-浓度由于吉非贝齐的相当大的CLO•反应性(1.93×10 9中号-1小号-1 ) 和萘普生 (9.24 × 10 9 M -1 s -1) 和 Cl 2 • −快速转化为 ClO•。由于 CO 3 • -的产生,HCO 3 -的存在显着促进了紫外线/氯工艺中的降解。CO 3 • -显示出与吉非贝齐和萘普生的高反应性,相应的二级反应速率常数分别为 2.45 × 10 7和 3.50 × 10 7 M -1 s -1. 溶解的有机物对•OH、Cl• 和ClO• 产生了明显的清除作用,并大大延缓了降解。考虑到上述水基体影响的构建模型成功预测了实际水中的氧化降解动力学,并且•OH 和CO 3 • -都是降解中的主要反应物质。该研究有助于全面了解可能的自由基物种在真实水基质下紫外线/氯氧化去除微污染物中的作用。


























 京公网安备 11010802027423号
京公网安备 11010802027423号